Advanced Control in Cellular Therapies with Molecular Switch Technology
Introduction
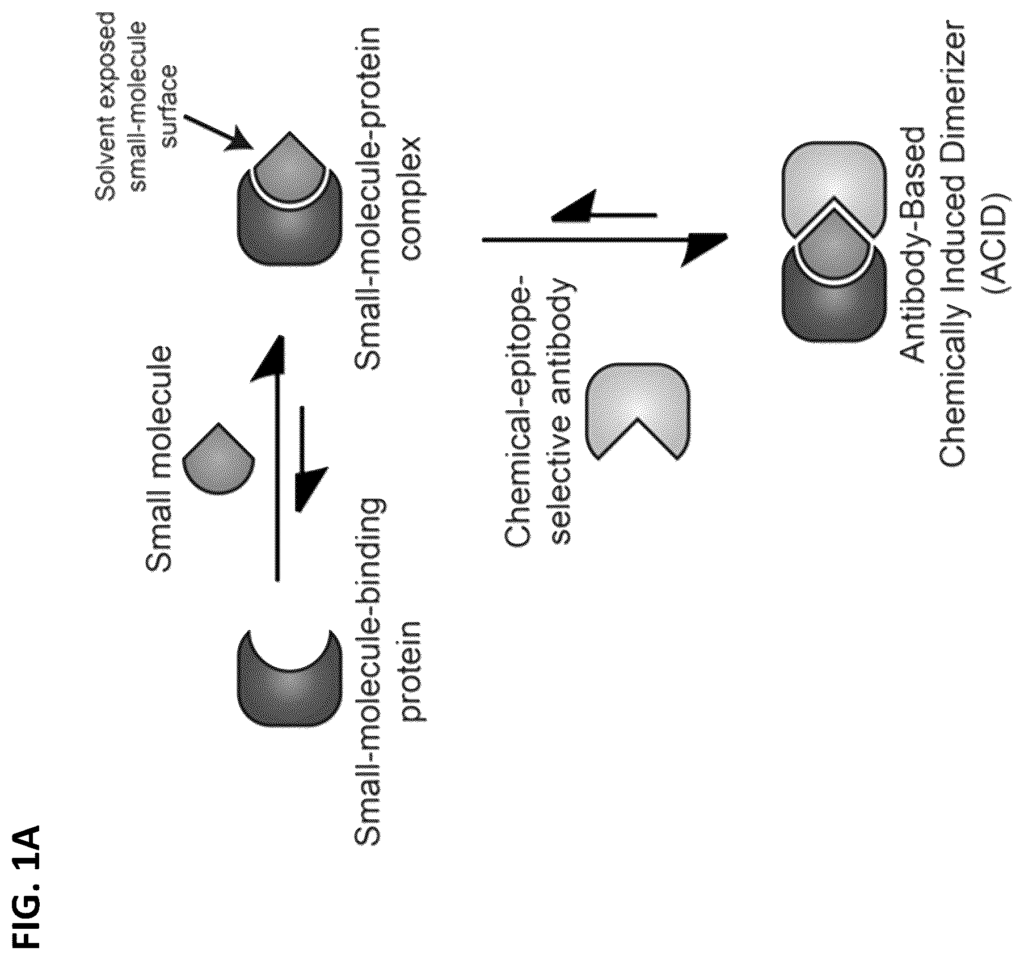
Cellular therapies hold immense promise for treating a wide array of diseases, from cancer to autoimmune disorders. However, one of the biggest challenges with cellular therapies is the ability to precisely control therapeutic activity, ensuring it targets only diseased cells without affecting healthy tissues. Our patented Antibody Chemically Induced Dimerizer (AbCID) technology offers a novel solution for regulating cellular therapies with unprecedented precision. This technology enables researchers and healthcare providers to activate or deactivate specific cellular therapies on demand, improving safety, efficacy, and flexibility.
Limitations of Current Cellular Therapies
While cellular therapies, such as CAR-T cells, have shown remarkable success in treating certain cancers, the challenge remains in controlling these therapies once they are introduced into the body. Current methods often lack the precision needed to prevent unintended side effects, such as immune overactivation or off-target damage to healthy cells. Without the ability to regulate these therapies in real-time, patients face the risk of severe adverse reactions, which can compromise treatment success.
There is a growing need for a technology that allows healthcare providers to fine-tune cellular therapies, ensuring that they remain effective while minimizing potential harm to the patient.
A New Approach to Cellular Therapy Control
Our AbCID technology offers an innovative molecular switch that can control cellular therapies with chemical precision. By introducing a dimerizer system that is activated by specific antibodies, this technology allows for the real-time regulation of therapeutic activity at the molecular level. The dimerizer can activate or deactivate cellular functions, making it possible to switch the therapy on or off based on the patient’s response. This provides an added layer of safety, ensuring that therapies are only active when needed and reducing the risk of unintended side effects.
This system is highly adaptable and can be integrated into a wide range of cellular therapies, including cancer treatments, gene therapies, and immune-modulating therapies. By offering precision control over therapeutic action, AbCID enables more personalized and responsive treatments for patients, improving both efficacy and safety.
Key Benefits
- Precision Control: Allows for on-demand activation and deactivation of cellular therapies, ensuring precise targeting of diseased cells.
- Enhanced Safety: Reduces the risk of adverse side effects by providing real-time regulation of therapeutic activity.
- Broad Applicability: Can be applied to a variety of cellular therapies, including cancer, autoimmune, and genetic treatments.
- Improved Patient Outcomes: Enables more tailored, responsive therapies that can be adjusted based on patient needs.
Transforming the Future of Cellular Therapies
Licensing this molecular switch technology offers pharmaceutical companies and biotechnology firms a cutting-edge tool for advancing cellular therapies. With its ability to provide precision control over therapeutic activity, this technology opens new doors for safer, more effective treatments in oncology, immunotherapy, and beyond.
- Abstract
- Claims
What is claimed is:
1. A system comprising:
10. A system comprising:
11. A system comprising:
Share
Title
Antibody chemically induced dimerizer (AbCID) as molecular switches for regulating cellular therapies
Inventor(s)
James A Wells, Zachary B. HILL, Alexander J. MARTINKO
Assignee(s)
University of California, Antheia Inc
Patent #
11939379
Patent Date
March 26, 2024
