Targeted Prodrug Therapy for Cancer and Neurodegenerative Diseases
Introduction
Cancer and neurodegenerative diseases remain two of the most challenging health conditions to treat due to their complexity and resistance to traditional therapies. Recent advancements in the development of proteasome inhibitors, which regulate protein degradation in cells, have shown great promise in addressing both cancer progression and neurodegenerative disorders. Our patented proteasome-inhibiting β-lactam prodrugs offer a targeted and effective approach to treating these diseases, providing pharmaceutical companies and researchers with a novel solution for creating life-saving therapies.
Addressing the Treatment Gaps in Cancer and Neurodegenerative Diseases
Cancer therapies have evolved over the years, yet many treatments still fall short in targeting tumor cells with precision while minimizing damage to healthy tissues. Additionally, neurodegenerative disorders like Alzheimer’s and Parkinson’s lack effective treatments that can stop or reverse the progression of the disease. Existing medications often focus on managing symptoms rather than addressing the underlying cellular dysfunction that drives these conditions.
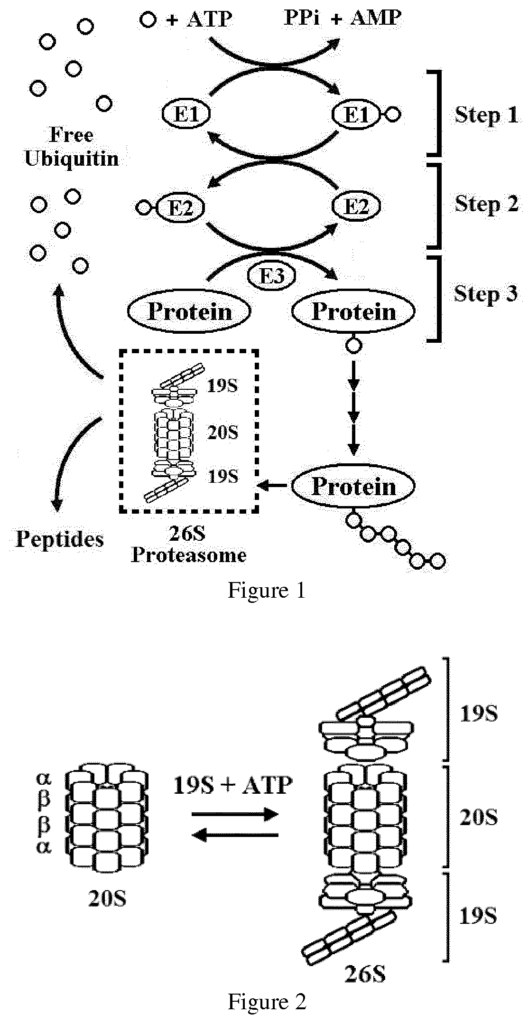
The use of proteasome inhibitors has emerged as a promising therapeutic strategy, as proteasomes play a key role in regulating protein degradation in cells. Disrupting this process in cancer cells can lead to cell death, making it an effective anti-tumor strategy. Similarly, targeting proteasome activity in neurodegenerative diseases can help restore proper protein balance, potentially slowing or stopping disease progression. However, developing treatments that can deliver these inhibitors with high specificity and minimal side effects is a critical challenge.
A Targeted Therapeutic Approach with β-Lactam Prodrugs
Our proteasome-inhibiting β-lactam prodrugs introduce a breakthrough approach to targeting and inhibiting proteasome activity in diseased cells. These prodrugs are designed to remain inactive until they reach the target site, where they are converted into active inhibitors within the cancerous or neurodegenerative cells. This ensures high specificity and reduces the risk of systemic side effects, making the treatment both effective and safer for patients.
The prodrugs are especially valuable in treating cancer, as proteasome inhibition disrupts the regulatory systems that cancer cells rely on to survive and proliferate. Similarly, in neurodegenerative diseases, this approach helps manage the accumulation of misfolded proteins that lead to cell damage. By leveraging the selective activation of the prodrugs, this technology offers a precise method for controlling proteasome activity and improving patient outcomes in both oncology and neurology.
Key Benefits
- Targeted Therapy: Delivers proteasome inhibitors directly to diseased cells, reducing off-target effects and improving treatment efficacy.
- Versatile Applications: Effective in treating both cancer and neurodegenerative diseases, offering a broad therapeutic scope.
- Reduced Side Effects: Prodrug design ensures that the active compound is only released in the target cells, minimizing systemic toxicity.
- Potential to Slow Disease Progression: In neurodegenerative diseases, proteasome inhibition can help restore protein balance and reduce cellular damage.
Transforming Treatment for Complex Diseases
Licensing this proteasome-inhibiting prodrug technology provides pharmaceutical companies with a powerful tool to develop next-generation therapies for cancer and neurodegenerative disorders. By offering a targeted approach to treatment, this technology opens new avenues for addressing some of the most challenging health conditions, bringing hope for improved patient outcomes.
- Abstract
- Claims
The invention claimed is:
Share
Title
Proteasome inhibiting β-lactam prodrugs useful for the treatment of cancer and neurodegenerative disorders
Inventor(s)
Philippe Yves-Rémy Simon, Henri Oreal, Gérard Audran, Marvin Schulz, Jean-Patrick Joly, Didier Siri, Anouk Siri
Assignee(s)
Philippe Yves Remy Simon, Aix Marseille Universite, Centre National de la Recherche Scientifique CNRS
Patent #
11053249
Patent Date
July 6, 2021








