Scalable Molecular Engineering with Self-Replicating DNA Origami
Introduction
In the field of nanotechnology, the ability to design and build complex molecular structures with precision is critical to unlocking new applications in areas such as drug delivery, diagnostics, and molecular computing. However, traditional fabrication methods can be costly, labor-intensive, and difficult to scale. Our patented self-replicating nucleic acid origami tiles offer a breakthrough in molecular engineering, enabling the autonomous and scalable replication of DNA-based structures. This innovation provides a versatile platform for researchers, biotechnologists, and pharmaceutical companies looking to create sophisticated molecular systems more efficiently.
Limitations in Traditional Molecular Fabrication
The concept of DNA origami—where DNA strands are folded into specific shapes and structures—has proven highly effective in the design of nanoscale devices. However, one of the biggest challenges in DNA origami and molecular engineering is scalability. Current methods require significant manual input for the construction of each individual structure, making it difficult to produce large quantities of complex molecules. For applications such as targeted drug delivery, biosensing, or molecular computing, the ability to mass-produce DNA-based devices is essential for both research and commercial deployment.
Moreover, traditional methods of molecular self-assembly often lack the ability to autonomously replicate the designed structures, which limits their use in dynamic environments that require adaptability and growth over time.
Self-Replicating DNA Origami: A Game-Changer for Molecular Systems
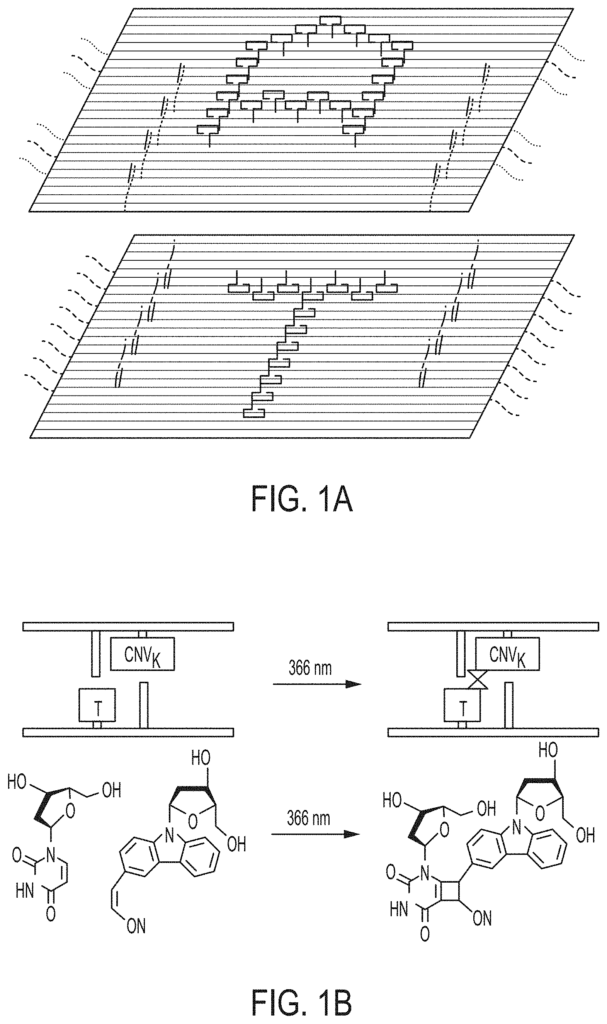
Our patented self-replication technology for nucleic acid origami tiles addresses these limitations by enabling the autonomous replication of designed DNA structures. Through a carefully engineered process, these origami tiles are programmed to self-replicate, generating identical copies of the initial structure. This self-replication ability not only enhances scalability but also allows for the continuous generation of complex molecular systems in real-time, making it an ideal solution for applications requiring large quantities of nanoscale devices.
The potential applications of this technology are vast. In the pharmaceutical industry, it can be used to create programmable drug delivery systems that replicate themselves as needed. In molecular diagnostics, it enables the development of dynamic biosensors that continuously produce signal-enhancing nanostructures. Additionally, in the field of synthetic biology, this system can be used to engineer self-replicating biological systems for advanced research and therapeutic applications.
Key Benefits
- Scalable Molecular Fabrication: Enables the mass production of DNA-based nanostructures through autonomous self-replication, reducing labor and time.
- Versatile Applications: Suitable for drug delivery systems, biosensing, molecular computing, and synthetic biology research.
- Cost-Effective: Self-replication reduces the need for manual input and complex assembly processes, lowering production costs.
- Dynamic Adaptability: Allows for the continuous generation of nanostructures, making it ideal for environments requiring real-time adaptability.
Building the Future of Molecular Engineering with Self-Replication
Licensing this self-replicating DNA origami technology offers companies in biotechnology, nanotechnology, and pharmaceuticals a cutting-edge tool for advancing molecular engineering. By enabling scalable, cost-effective, and autonomous replication of DNA structures, this innovation opens new doors for developing sophisticated molecular devices and systems that can impact a wide range of industries.
- Abstract
- Claims
What is claimed is:
1. A method for exponential self-replication of nucleic acid origami tiles, comprising:
13. The method of claim 1, wherein:
14. The method of claim 13, wherein:
15. The method of claim 13, wherein:
Share
Title
Self-replication of nucleic acid origami tiles
Inventor(s)
Xiaojin He, Ruojie Sha, Yongli Mi, Paul Chaikin, Nadrian C. Seeman
Assignee(s)
New York University NYU
Patent #
10513535
Patent Date
December 24, 2019