Advanced Estrogen Receptor Therapy for Cancer
Introduction
This innovative technology provides a targeted solution to address estrogen receptor-driven cancers by regulating estrogen receptor proteolysis. By focusing on estrogen receptor modulation, this technology supports the reduction of hormone-driven cancer growth, delivering a precision approach that offers efficacy with reduced side effects. For companies in biotechnology, oncology, and women’s health, this technology opens pathways to develop next-generation therapies for estrogen receptor-positive cancers, such as breast and ovarian cancer, meeting a critical need for safer, more effective treatments in the field of hormone-driven oncology.
The Challenge: Managing Estrogen Pathways in Cancer Treatment
Hormone-driven cancers are particularly challenging to treat, as they rely on the body’s own hormonal pathways to fuel tumor growth. Estrogen receptors in particular play a significant role in many types of cancers, such as breast cancer, where an overabundance of estrogen receptors can drive aggressive tumor growth. Traditional therapies attempt to block or reduce estrogen effects systemically, but these methods often lead to significant side effects and compromise the body’s natural hormonal balance. Patients need targeted therapies that specifically impact the cancer cells’ reliance on estrogen without impacting healthy tissue function.
Targeted Proteolysis of Estrogen Receptors
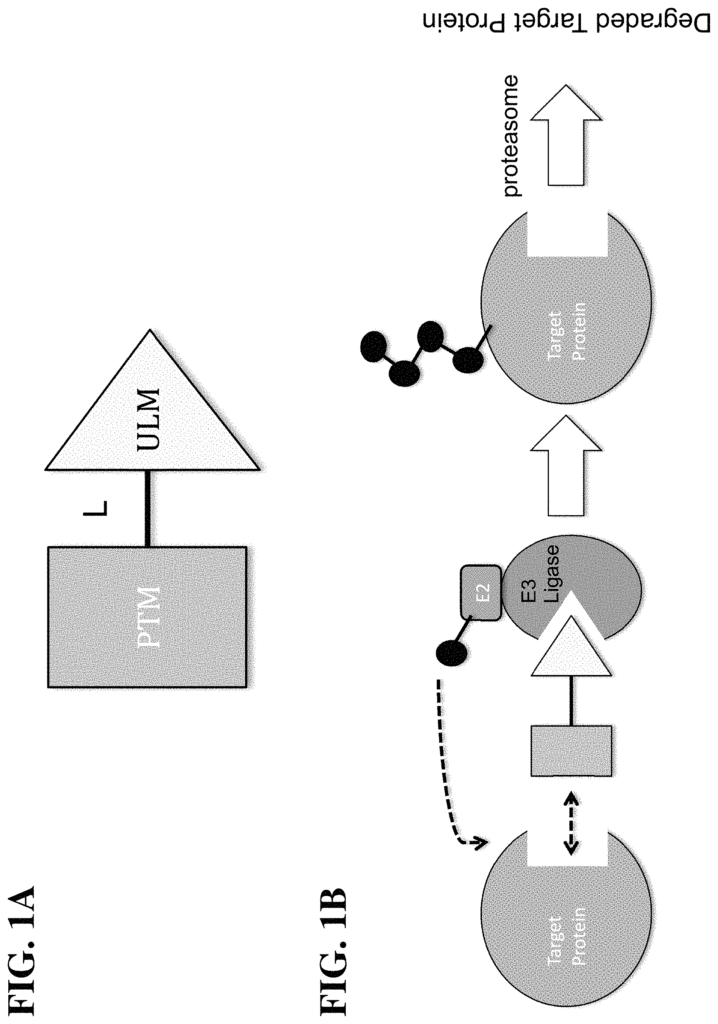
This advanced modulation technology offers a precise approach to reduce estrogen receptor activity in targeted cells by initiating receptor proteolysis, a natural process where specific proteins are broken down to reduce their activity. This selective degradation of estrogen receptors allows for a more controlled approach in disrupting cancer cell growth without the need for broad systemic hormone manipulation. By specifically targeting estrogen receptors in cancer cells, this approach minimizes off-target effects, preserving overall hormonal balance and reducing patient discomfort during treatment.
Key Benefits for Oncology and Hormone Therapy Fields
For pharmaceutical and biotechnology companies, this targeted estrogen receptor modulation technology represents a breakthrough approach to managing hormone-driven cancers. It provides a safer, more patient-centered alternative to conventional hormone therapies, addressing the urgent need for therapies that effectively inhibit tumor growth while minimizing side effects. Oncology centers, women’s health providers, and pharmaceutical developers can leverage this technology to provide patients with a more refined, reliable cancer treatment option. This technology meets the demand for specialized, precision treatments, positioning your company as a leader in advancing cancer care.
Invest in Targeted Cancer Treatment Innovation
Licensing this estrogen receptor modulation technology positions your company at the forefront of hormone-driven cancer therapy. By providing a targeted, effective solution to combat hormone-related cancers, your business can bring hope to patients and families affected by estrogen-driven tumors. This technology is a valuable asset for companies dedicated to advancing oncology, hormone therapy, and women’s health, offering an innovative path forward in the battle against cancer.
- Abstract
- Claims
What is claimed is:
CLM-L-PTM,
Share
Title
Modulators of estrogen receptor proteolysis and associated methods of use
Inventor(s)
Andrew P. Crew, Keith R. Hornberger, Hanqing Dong, Jing Wang, Yimin Qian, Craig M. Crews
Assignee(s)
Arvinas Operations Inc
Patent #
10604506
Patent Date
March 31, 2020



















































































