A Targeted Approach for Treating Neurodegenerative Diseases with nSMase2 Inhibitors
Introduction
Neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS), are among the most challenging conditions for the healthcare system. These diseases progressively damage nerve cells in the brain and spinal cord, leading to cognitive decline, motor function loss, and, ultimately, death. Current treatments offer only symptomatic relief and fail to address the underlying causes of these conditions. With the aging global population, the need for new, targeted therapeutic strategies has never been greater. Our patented small-molecule inhibitors of neutral sphingomyelinase 2 (nSMase2) provide an innovative approach to treating neurodegenerative diseases by targeting a key enzyme involved in neuroinflammation and cell death.
Limitations of Existing Treatments
Current therapies for neurodegenerative diseases primarily focus on symptom management, offering minimal impact on disease progression. These treatments often involve addressing secondary symptoms such as tremors or memory loss, without halting the disease itself. Additionally, many neurodegenerative diseases, like ALS and Alzheimer’s, still have no cure, and treatment options remain limited in their ability to slow down or reverse neurological damage. The healthcare sector is in dire need of a therapeutic approach that addresses the root causes of these diseases, providing real hope for long-term disease management and improved quality of life for patients.
A Novel Approach with nSMase2 Inhibitors
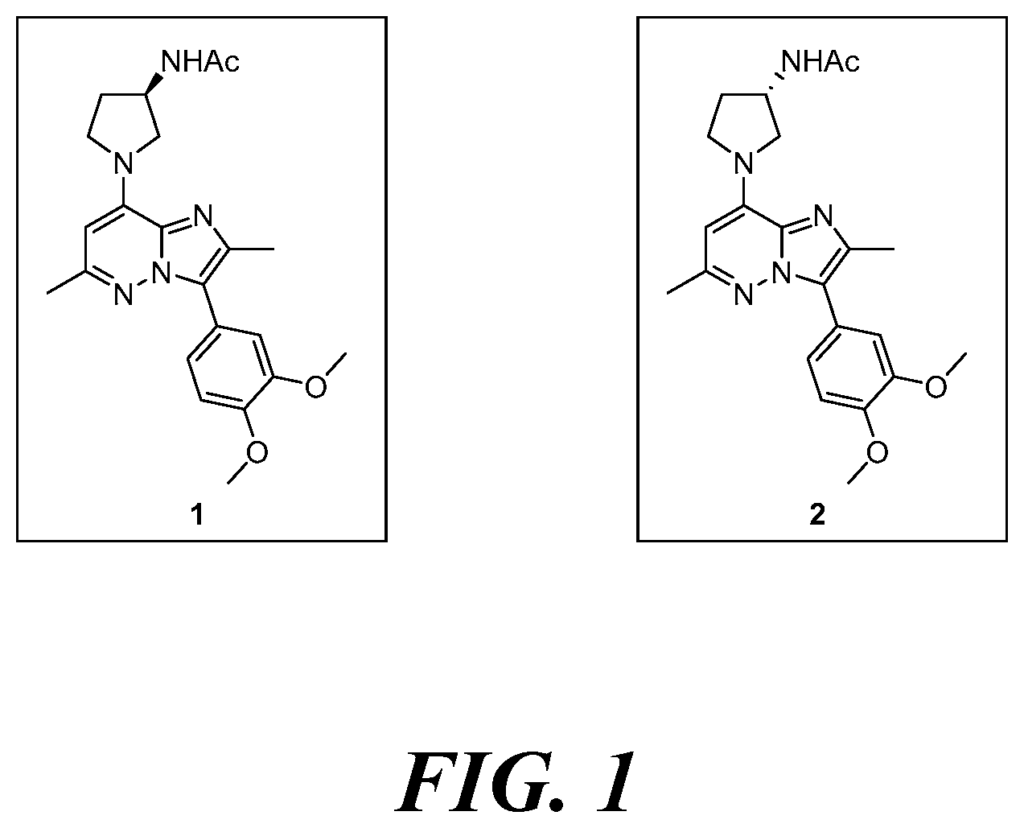
Our patented technology offers a breakthrough by targeting the enzyme neutral sphingomyelinase 2 (nSMase2), which is known to play a critical role in the progression of neurodegenerative diseases. By inhibiting nSMase2 activity, these small-molecule inhibitors reduce the production of ceramide, a lipid molecule involved in neuroinflammation and neuronal death. This approach aims to slow down or stop the neurodegenerative process, directly addressing the cellular mechanisms behind these diseases.
This therapy offers the potential for disease-modifying effects, giving patients more than just temporary relief from symptoms. Instead, it targets the biological pathways that contribute to neurodegeneration, offering hope for slowing disease progression and possibly preventing further neuronal damage.
Advantages of Licensing This Technology
- Targeted Mechanism: The inhibition of nSMase2 addresses the cellular processes leading to neurodegeneration, potentially slowing disease progression.
- Broader Therapeutic Applications: While initially focused on Alzheimer’s, Parkinson’s, and ALS, this approach could have broader applications for other neurodegenerative conditions.
- Disease-Modifying Potential: Unlike current treatments that only manage symptoms, this technology offers the possibility of slowing or halting the disease process at its core.
- Reduced Inflammation: By targeting neuroinflammation, these inhibitors could improve overall brain health and long-term patient outcomes.
A Pivotal Opportunity in Neurodegenerative Disease Treatment
Licensing this small-molecule inhibitor technology positions your company to be at the forefront of neurotherapeutic innovation. As the demand for effective neurodegenerative treatments continues to rise, this patent offers a pathway to providing patients and healthcare providers with new hope for managing and potentially altering the course of these devastating diseases.
- Abstract
- Claims
That which is claimed:
Share
Title
Small molecule inhibitors of neutral sphingomyelinase 2 (nSMase2) for the treatment of neurodegenerative diseases
Inventor(s)
Barbara Slusher, Camilo Rojas, Ajit G. Thomas, Radim Nencka, Michal Sala, Hubert Hrebabecky, Norman Haughey
Assignee(s)
Johns Hopkins University, Institute of Organic Chemistry and Biochemistry of ASCR vvi
Patent #
11427590
Patent Date
August 30, 2022