Innovative Lung Repair with Advanced Hydrogel Sealant Technology
Introduction
Lung injuries, whether caused by trauma, surgery, or disease, present significant challenges for both patients and healthcare providers. Traditional methods of lung tissue repair often involve mechanical sealing techniques or synthetic adhesives, which can lead to complications such as poor integration with the tissue, inflammation, or insufficient healing. Our patented cell-seeded porous lung hydrogel sealant offers a groundbreaking approach to tissue repair, utilizing cutting-edge biotechnology to improve outcomes and facilitate more effective recovery in patients with lung injuries or conditions.
The Need for Enhanced Lung Repair Solutions
Lung injuries can be difficult to treat due to the delicate nature of lung tissue and its critical role in oxygen exchange. Current treatments, such as mechanical sutures or synthetic sealants, often struggle to provide a reliable and biocompatible solution for sealing lung tissue. These methods can lead to issues like air leaks, prolonged healing times, or the risk of infection. Additionally, the lung’s constant movement during breathing makes it challenging for traditional methods to remain effective over time, creating a need for a more dynamic and biologically compatible solution.
Advanced Hydrogel Technology for Lung Tissue Repair
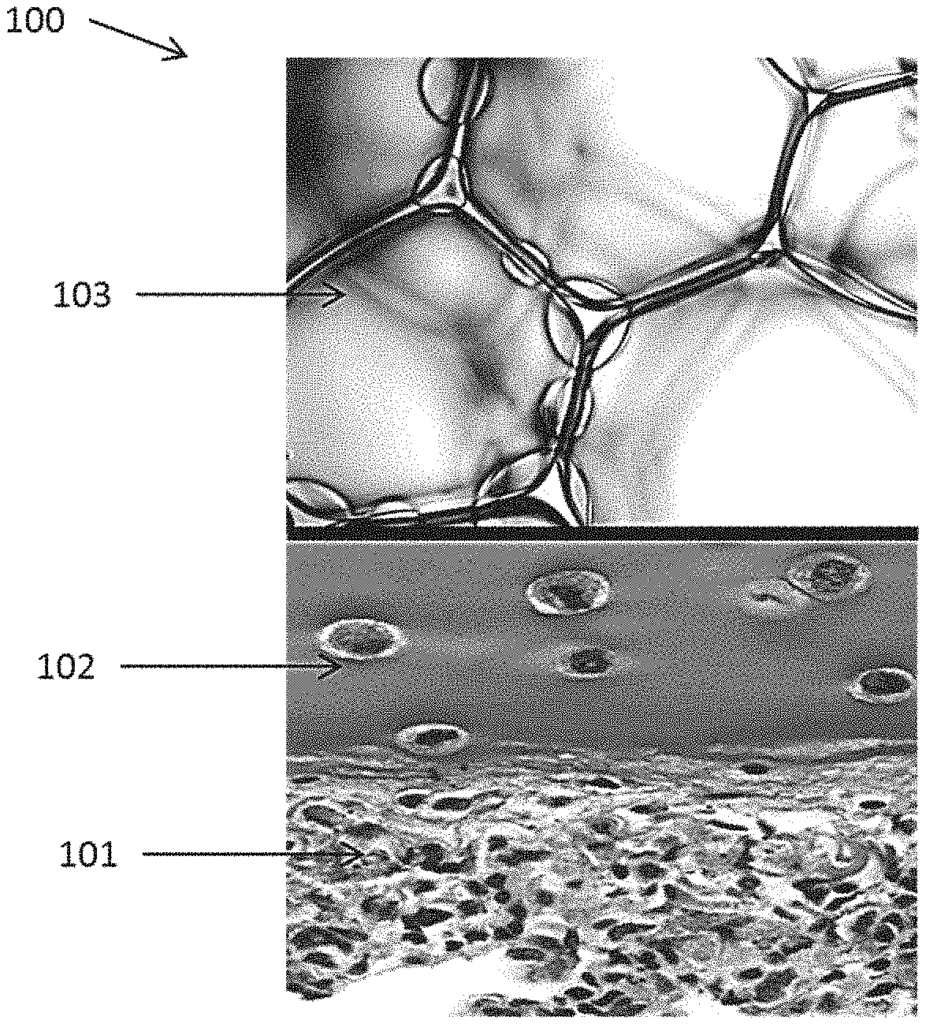
Our cell-seeded porous lung hydrogel sealant addresses these challenges by providing a bioengineered solution that promotes tissue regeneration while effectively sealing lung injuries. The hydrogel is seeded with cells that aid in the healing process, allowing for better integration with the surrounding lung tissue. Its porous structure enables the hydrogel to conform to the dynamic movements of the lung, providing a flexible and durable seal. This innovation not only reduces the risk of air leaks but also enhances the overall healing process by supporting tissue regeneration at the injury site.
The cell-seeded hydrogel promotes biocompatibility, reducing the risk of inflammation or rejection while creating an environment conducive to natural lung tissue growth. This technology is particularly suited for use in pulmonary surgeries, trauma care, and lung disease treatment, offering a highly effective and biologically friendly option for patients in need of lung repair.
Advantages of This Lung Hydrogel Sealant
- Improved Biocompatibility: The cell-seeded hydrogel integrates with the surrounding lung tissue, reducing complications such as inflammation and promoting faster healing.
- Flexibility and Durability: Its porous structure allows the hydrogel to conform to lung movements, providing a reliable and long-lasting seal.
- Tissue Regeneration: The inclusion of cells within the hydrogel supports tissue growth, enhancing the body’s natural healing response.
- Broad Medical Applications: Ideal for use in lung surgery, trauma care, and treating pulmonary conditions, this technology offers versatility across the medical field.
A Leap Forward in Lung Healing
Licensing this innovative lung hydrogel sealant technology offers healthcare providers and medical device manufacturers an opportunity to advance the field of pulmonary care. By combining biocompatibility with enhanced tissue regeneration capabilities, this technology provides a critical solution for addressing lung injuries, improving patient outcomes, and reducing the risks associated with traditional repair methods.
- Abstract
- Claims
What is claimed is:
1. A biosealant composition comprising an extracellular matrix hydrogel and a thermogel having pores, the pores having an average diameter of about 0.025 mm to about 0.90 mm;
11. A method of making the biosealant composition according to claim 1 comprising the steps of:
14. A biosealant composition comprising an extracellular matrix hydrogel and a thermogel comprising a gelatinous material and a cross-linking enzyme in an amount sufficient to result in gelation of the thermogel at a temperature from about 35° C. to 37° C., wherein the thermogel has pores of average diameter of about 0.025 mm to 0.90 mm;
21. A biosealant composition comprising an extracellular matrix hydrogel comprising
Share
Title
Cell-seeded porous lung hydrogel sealant
Inventor(s)
Matthew Bacchetta, Brandon Guenthart, Jinho Kim, John O'Neill, Gordana Vunjak-Novakovic
Assignee(s)
Columbia University in the City of New York
Patent #
11291747
Patent Date
April 5, 2022