Advancing Alzheimer’s Research with Stable Beta-Amyloid Oligomers
Introduction
Neurodegenerative diseases, particularly Alzheimer’s, represent a significant challenge in modern medicine. One of the key factors in these diseases is the accumulation of beta-amyloid plaques in the brain, a process driven by amyloid oligomers. While much research has focused on these plaques, targeting oligomers directly could offer a new therapeutic pathway. Our patented synthetic beta-amyloid peptides capable of forming stable antigenic oligomers open new doors for Alzheimer’s research, diagnostics, and treatment, offering a highly targeted approach to managing amyloid aggregation disorders.
The Urgent Need for Innovative Alzheimer’s Treatments
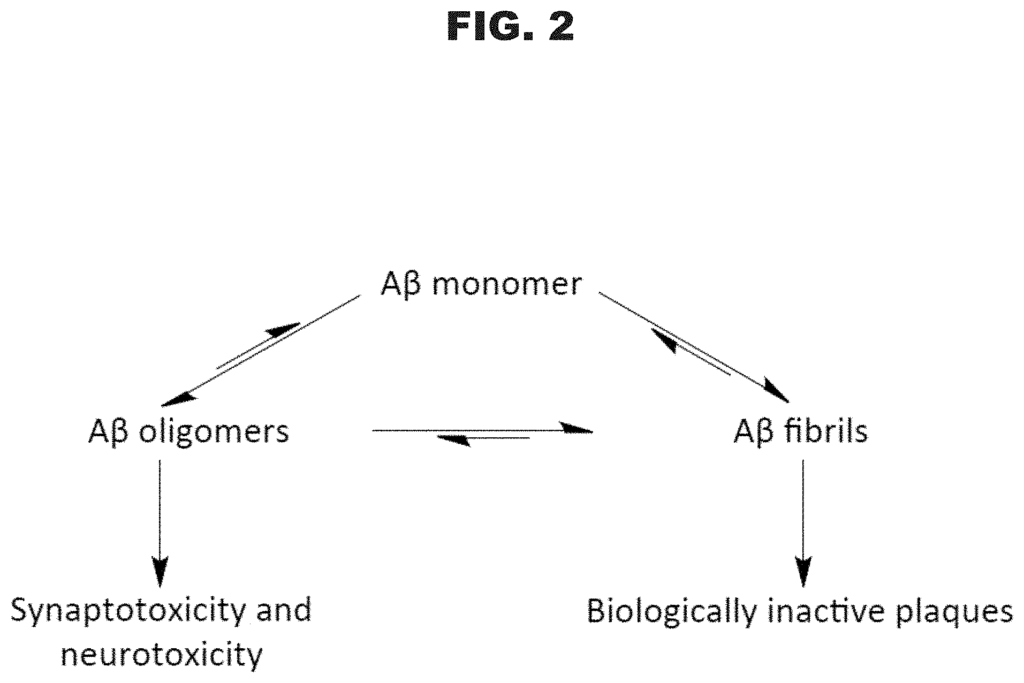
Alzheimer’s disease remains one of the most complex and devastating neurodegenerative conditions, affecting millions of people worldwide. Current treatments focus on symptom management rather than addressing the underlying cause of the disease—namely, the misfolding and aggregation of beta-amyloid peptides into toxic oligomers and plaques. Despite decades of research, there is still no cure, and effective therapies are limited.
Researchers and pharmaceutical companies face the challenge of developing treatments that can slow or stop disease progression by targeting these amyloid oligomers, which are believed to be more toxic than the plaques themselves. Additionally, developing diagnostic tools to detect these oligomers early could drastically improve patient outcomes by enabling earlier intervention.
Targeting Beta-Amyloid Oligomers with Stability and Precision
Our patented synthetic beta-amyloid peptides offer a breakthrough in this area by forming stable antigenic oligomers that closely mimic the toxic species found in neurodegenerative diseases like Alzheimer’s. These peptides can be used as a model for studying the mechanisms of amyloid aggregation and provide a critical tool for developing diagnostics, vaccines, and treatments. The stable nature of these oligomers makes them ideal for drug screening, allowing researchers to test compounds that could inhibit amyloid formation or enhance clearance from the brain.
Furthermore, these synthetic oligomers can be used in diagnostic assays to detect early amyloid aggregation, enabling the development of blood tests or other non-invasive methods for diagnosing Alzheimer’s before symptoms fully develop. This could significantly enhance early intervention strategies.
Key Benefits
- Therapeutic Development: Enables the creation of drugs and vaccines targeting toxic amyloid oligomers in Alzheimer’s and related diseases.
- Stable and Reproducible: Provides a reliable model for studying amyloid aggregation and testing potential inhibitors.
- Diagnostic Potential: Can be used in diagnostic assays to detect amyloid aggregation early, improving the chances of early intervention.
- Broad Application: Valuable for research, drug discovery, and diagnostics related to neurodegenerative diseases.
A New Avenue for Alzheimer’s Research and Treatment
Licensing this synthetic beta-amyloid peptide technology provides pharmaceutical companies and research institutions with a powerful tool to accelerate the development of new diagnostics and therapies for Alzheimer’s disease. This technology represents a key advance in targeting the toxic oligomers that drive neurodegeneration.
- Abstract
- Claims
What is claimed is:
1. A method of producing antibodies having affinity for soluble beta-amyloid oligomers comprising:
wherein the beta-amyloid trimer is a trimer selected from the group consisting of:
a trimer having a first, a second, and a third synthetic beta-amyloid peptide, each peptide comprises Seq. ID No. 3 or a substantially similar sequence;
a trimer having a first, a second, and a third synthetic beta-amyloid peptide, each peptide comprises Seq. ID No. 4 or a substantially similar sequence;
a trimer having a first, a second, and a third synthetic beta-amyloid peptide, each peptide consists of a first and a second strand, the first strand comprises Seq. ID No. 5 or a substantially similar sequence, and the second strand comprises Seq. ID No. 6 or a substantially similar sequence;
a trimer having a first, a second, and a third synthetic beta-amyloid peptide, each peptide consists of a first and a second strand, the first strand comprises Seq. ID No. 7 or a substantially similar sequence, and the second strand comprises Seq. ID No. 8 or a substantially similar sequence;
Share
Title
Synthetic beta-amyloid peptides capable of forming stable antigenic oligomers
Inventor(s)
James S. Nowick, Adam G. Kreutzer, Ryan K. Spencer, Patrick J. Salveson
Assignee(s)
University of California
Patent #
11319348
Patent Date
May 3, 2022