Innovative Antigen Display with Self-Assembling Ferritin Nanoparticles
Introduction
In the rapidly evolving fields of immunotherapy and vaccine development, ensuring the effective delivery and display of antigens is crucial for stimulating robust immune responses. Traditional methods of antigen delivery often face challenges in ensuring proper assembly, stability, and targeting. Our patented technology offers a breakthrough solution through self-assembling ferritin nanoparticles derived from insect proteins. These nanoparticles are designed to co-assemble with trimeric antigens, providing an innovative platform for enhanced antigen presentation and improved immune responses in vaccines and immunotherapies.
Current Limitations in Antigen Delivery and Display Systems
One of the main hurdles in developing effective vaccines and immunotherapies is finding a reliable delivery method that ensures the proper presentation of antigens to the immune system. Conventional approaches sometimes struggle with antigen stability, appropriate folding, and achieving high immunogenicity. These limitations can reduce the effectiveness of the vaccine or therapeutic treatment, particularly when dealing with complex antigens that require specific configurations to elicit a strong immune response.
In the pharmaceutical and biotechnology industries, there is a growing demand for advanced delivery platforms that can overcome these challenges, providing stable, consistent, and immunogenic antigen display systems. Nanoparticles have emerged as a promising solution for improving antigen delivery, but the development of self-assembling systems that can co-display multiple antigens remains a cutting-edge area of research.
Self-Assembling Nanoparticles for Effective Antigen Display
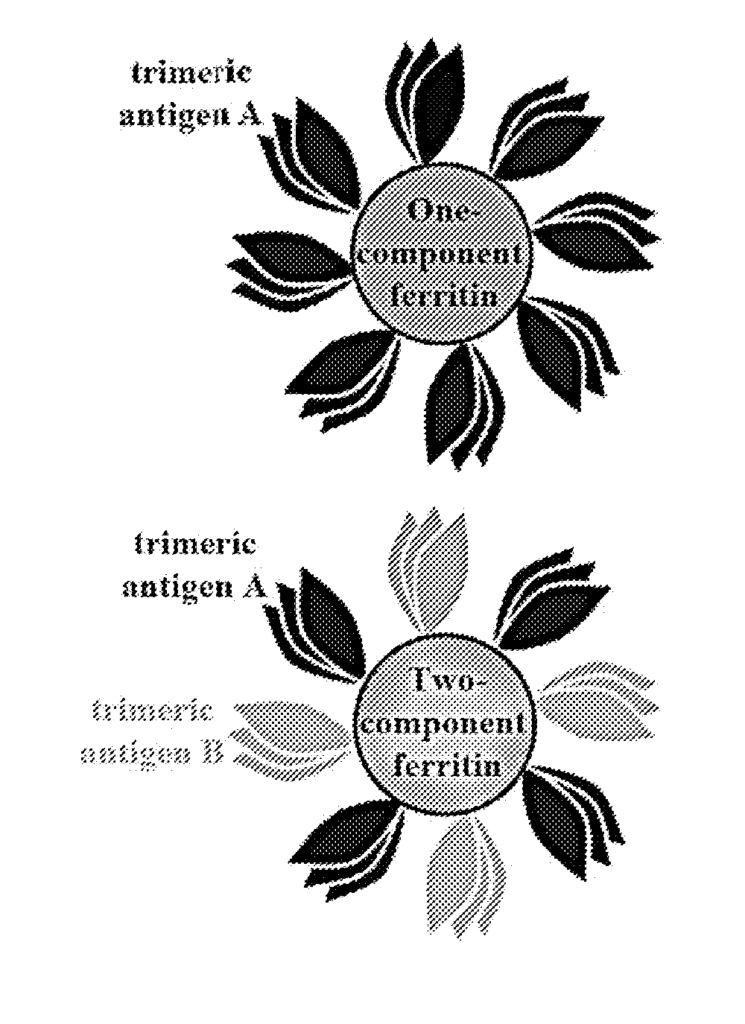
Our patented system introduces self-assembling insect ferritin nanoparticles as an innovative platform for displaying co-assembled trimeric antigens. Ferritin, a naturally occurring protein complex, has the unique ability to form nanoparticles that are stable, biocompatible, and highly structured. By leveraging ferritin derived from insect sources, this technology enables the assembly of nanoparticles that efficiently present trimeric antigens, ensuring that the antigens are correctly folded and displayed in a highly immunogenic configuration.
The self-assembling nature of the system ensures that the nanoparticles are produced consistently and reliably, providing a scalable solution for vaccine production. This technology has broad applications in the development of next-generation vaccines for infectious diseases, cancer immunotherapies, and other therapeutic areas that require precise antigen delivery and robust immune responses. Additionally, the use of trimeric antigens allows for the targeting of more complex pathogens and diseases, improving the efficacy of the treatment.
Key Benefits
- Enhanced Antigen Presentation: Provides a stable and structured platform for displaying trimeric antigens, improving immune responses.
- Self-Assembling Nanoparticles: Offers a reliable and scalable system for consistent nanoparticle production.
- Broad Therapeutic Applications: Suitable for vaccine development, cancer immunotherapy, and other immunological treatments.
- Improved Stability and Immunogenicity: Ensures that antigens are correctly folded and presented in an immunogenic configuration.
Empowering the Next Generation of Vaccines and Immunotherapies
Licensing this self-assembling nanoparticle technology provides pharmaceutical and biotechnology companies with a powerful tool for advancing vaccine development and immunotherapy. With its unique ability to present antigens in a structured and stable format, this system opens new possibilities for creating highly effective, next-generation treatments for a wide range of diseases.
- Abstract
- Claims
1. A recombinant insect ferritin nanoparticle, comprising:
3. The recombinant insect ferritin nanoparticle of claim wherein:
4. The recombinant insect ferritin nanoparticle of claim 1, wherein:
5. The recombinant insect ferritin nanoparticle of claim 1, wherein:
10. The recombinant insect ferritin nanoparticle of claim 6, wherein the first and second viral envelope protein ectodomains comprise:
11. The recombinant insect ferritin nanoparticle of claim 10, wherein the first and second viral envelope protein ectodomains comprise:
13. The recombinant insect ferritin nanoparticle of claim 10, wherein the first and second viral envelope protein ectodomains comprise HIV-1 Env ectodomains from two different strains of HIV-1, and wherein:
14. The recombinant insect ferritin nanoparticle of claim 10, wherein the first and second viral envelope protein ectodomains comprise influenza HA ectodomains or recombinant influenza HA stems from two different strains of influenza, and wherein:
16. The recombinant insect ferritin nanoparticle of claim 10, wherein the first and second viral envelope protein ectodomains comprise RSV F ectodomains from two different strains of RSV, and wherein:
20. A recombinant insect ferritin nanoparticle, comprising:
21. The recombinant insect ferritin nanoparticle of claim 20, wherein
22. An isolated nucleic acid molecule encoding:
23. An isolated nucleic acid molecule encoding:
27. A method of producing a recombinant insect ferritin nanoparticle, comprising:
32. The method of claim 29, wherein the recombinant insect ferritin nanoparticle comprises trimeric antigens comprising:
Share
Title
Self-assembling insect ferritin nanoparticles for display of co-assembled trimeric antigens
Inventor(s)
Peter Kwong, Ivelin Georgiev, Michael Gordon Joyce, Masaru Kanekiyo, Aliaksandr Druz, Ulrich Baxa, Joseph Van Galen, Rita Chen, Cheng Cheng, John Mascola, Yaroslav Tsybovsky, YongPing Yang, Paul Thomas, Barney Graham, Syed Mohammad Moin, Jeffrey Boyington, Kizzmekia Corbett
Assignee(s)
US Department of Health and Human Services
Patent #
20190330279
Patent Date
October 31, 2019