Enhance Chemical Reactions with Magnetic Fields
Introduction
In the complex world of chemical reactions, controlling reaction rates is crucial to ensure optimal efficiency, safety, and cost-effectiveness. From pharmaceuticals to energy production, industries rely on precise chemical processes to create high-quality products. Our patented method of using magnetic fields to control reaction rates offers a groundbreaking solution, providing enhanced control, improved efficiency, and greater flexibility in various chemical processes.
Challenges in Controlling Chemical Reactions
In many industries, chemical reactions are the backbone of production processes. Whether it’s the synthesis of new materials, the development of pharmaceutical compounds, or the conversion of energy, the ability to control reaction rates is essential. Traditionally, reaction rates are manipulated through temperature, pressure, or the use of catalysts. However, these methods can be inefficient, costly, or limited in effectiveness, especially when dealing with complex or sensitive reactions.
Without precise control over these reactions, companies face challenges such as wasted resources, suboptimal product quality, and increased production costs. For industries that depend on chemical reactions, finding innovative ways to improve control and efficiency is a priority.
Why Choose Magnetic Field-Driven Reaction Control?
Our patented method offers a transformative approach by applying magnetic fields to control the rate of chemical reactions. This technology provides an unprecedented level of precision and flexibility, allowing industries to fine-tune reaction speeds without the need for excessive heat, pressure, or expensive catalysts. The magnetic field interacts with specific materials or reactants, altering their behavior in a way that enhances efficiency and performance.
For the pharmaceutical industry, this can mean more efficient drug production with higher yields and fewer byproducts. In energy applications, controlling reaction rates can lead to improved energy conversion and storage systems, contributing to more sustainable energy solutions. Across all sectors, the ability to manipulate reactions with this method offers significant cost savings, greater process safety, and enhanced product quality.
Key Benefits
- Precise Control: Adjust reaction rates with magnetic fields for greater precision.
- Improved Efficiency: Optimizes reactions, reducing waste and improving resource utilization.
- Cost Savings: Reduces the need for expensive catalysts or extreme conditions.
- Versatile Applications: Applicable to a wide range of industries, from pharmaceuticals to energy.
Optimize Your Chemical Processes with Magnetic Control
Licensing this technology allows industries to take advantage of a novel method for controlling chemical reaction rates. By applying magnetic fields, companies can improve the efficiency, safety, and scalability of their chemical processes, ensuring high-quality products and more sustainable operations.
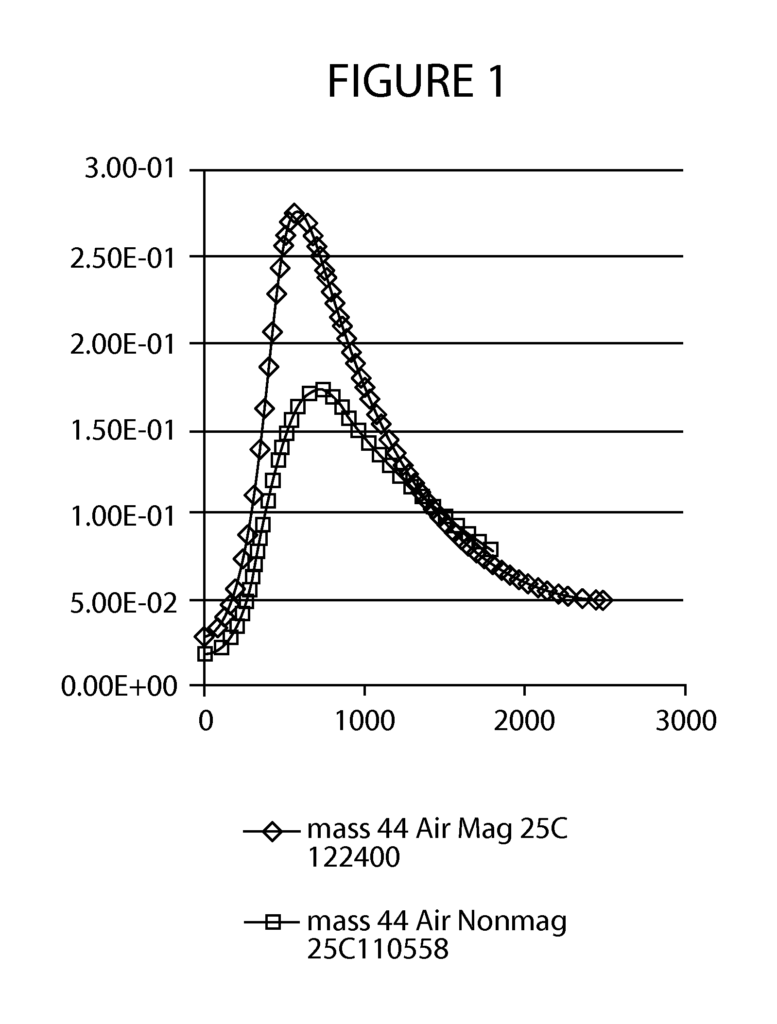
- Abstract
- Claims
We claim:
1. A method of promoting a chemical reaction, the method comprising the steps of:
21. A method of controlling a reaction rate of a chemical reaction, the method comprising:
29. An apparatus configured to effect a chemical reaction during which the reaction is exposed to a magnetic field, the apparatus comprising:
31. A method of increasing a reaction rate of a chemical reaction, the method comprising:
Share
Title
Methods and apparatus to control reaction rates of chemical reactions by applying a magnetic field
Inventor(s)
Reginald B. Little, James W. Mitchell
Assignee(s)
Howard University
Patent #
9511343
Patent Date
December 6, 2016