Transformative and Innovative CAR-T Cell Expansion for Cancer Care
Introduction
This groundbreaking CAR-T cell expansion technology provides a method to increase the production of antigen-specific CAR-T cells, offering a powerful tool for enhancing the effectiveness of CAR-T cell therapies in cancer treatment. By focusing on expanding specific CAR-T cells, this technology allows for more potent immune responses, improving targeting and efficacy against cancerous cells while minimizing off-target effects. For pharmaceutical and biotech companies, this technology represents an essential advancement in immunotherapy, providing a means to boost treatment outcomes, support patient recovery, and meet the growing demand for personalized cancer care.
The Challenge: Boosting Efficacy in CAR-T Therapies
As CAR-T therapies gain prominence in treating certain cancers, the ability to produce sufficient quantities of highly targeted, antigen-specific CAR-T cells remains a critical challenge. Current methods may limit the availability or potency of CAR-T cells, which can impact treatment efficacy, especially for patients with aggressive or resistant cancers. Healthcare providers and researchers need efficient methods to expand these cells to deliver robust, effective therapies, supporting long-term success in cancer management while minimizing adverse reactions.
Precise CAR-T Cell Expansion for Targeted Cancer Treatment
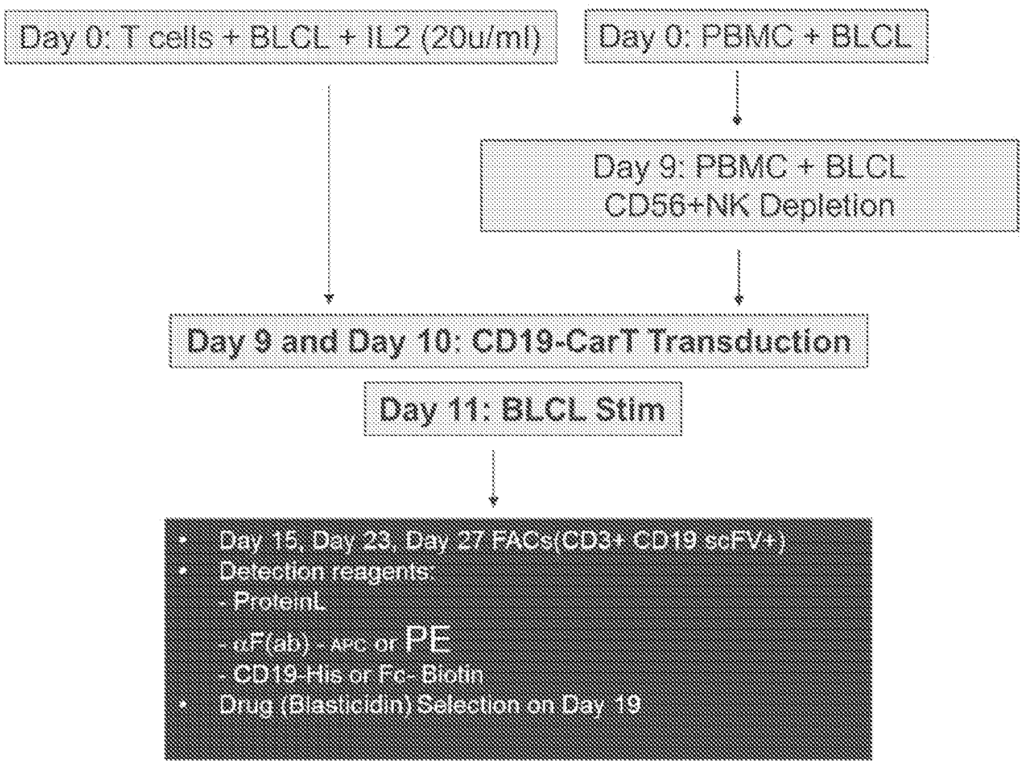
This technology offers a precise solution to these challenges by employing a method that promotes the rapid and targeted expansion of CAR-T cells specific to cancer antigens. By focusing on antigen-specific CAR-T cells, this method strengthens the body’s immune response against cancer cells while maintaining accuracy and reducing potential side effects. The technology provides a scalable and efficient means to increase CAR-T cell quantities, enabling clinicians to deliver potent, tailored treatments that address the unique requirements of each patient. This enhancement supports faster recoveries, improved survival rates, and more accessible CAR-T therapies.
Key Benefits for Pharmaceuticals and Immunotherapy Innovators
For pharmaceutical companies, this CAR-T cell expansion method opens up opportunities to develop advanced immunotherapies that offer higher efficacy with fewer side effects. Immunotherapy centers and oncology departments can integrate this technology into their treatment protocols, ensuring they can meet patient demands with powerful, scalable CAR-T therapies. The technology’s focus on antigen specificity and scalability aligns with the needs of personalized medicine, enabling providers to offer individualized care that maximizes therapeutic effectiveness while protecting healthy cells. This technology is poised to set new standards in immunotherapy, especially in areas where precision and potency are paramount.
Invest in the Future of Precision Cancer Immunotherapy
Licensing this CAR-T cell expansion technology positions your company as a leader in advanced cancer treatment solutions. By offering an effective, scalable approach to expanding antigen-specific CAR-T cells, your business can contribute to the future of immunotherapy, enhancing patient outcomes and supporting the broader goals of precision oncology. This technology is an invaluable asset for companies dedicated to transforming cancer care, promoting innovation in immunotherapy, and improving the lives of patients battling cancer.
- Abstract
- Claims
Share
Title
Methods for expanding antigen-specific car-t cells, compositions and uses related thereto
Inventor(s)
Blake T. AftabChristina PhamRhine ShenMichelle Wu
Assignee(s)
Atara Biotherapeutics Inc
Patent #
20210301255
Patent Date
September 30, 2021