Breakthrough Tumor Flare Management for Immunotherapy
Introduction
This innovative tumor flare management technology provides an essential method for controlling and mitigating the adverse effects associated with adoptive immunotherapy, a promising treatment approach for various cancers. By addressing the risk of tumor flare—an immune response that can cause temporary tumor growth and increased symptoms—this technology enables patients to experience the benefits of immunotherapy with reduced discomfort and complications. For companies in oncology, immunotherapy, and pharmaceuticals, this technology represents a critical asset, enhancing patient outcomes and expanding access to transformative cancer treatments.
The Challenge: Managing Tumor Flare in Immunotherapy
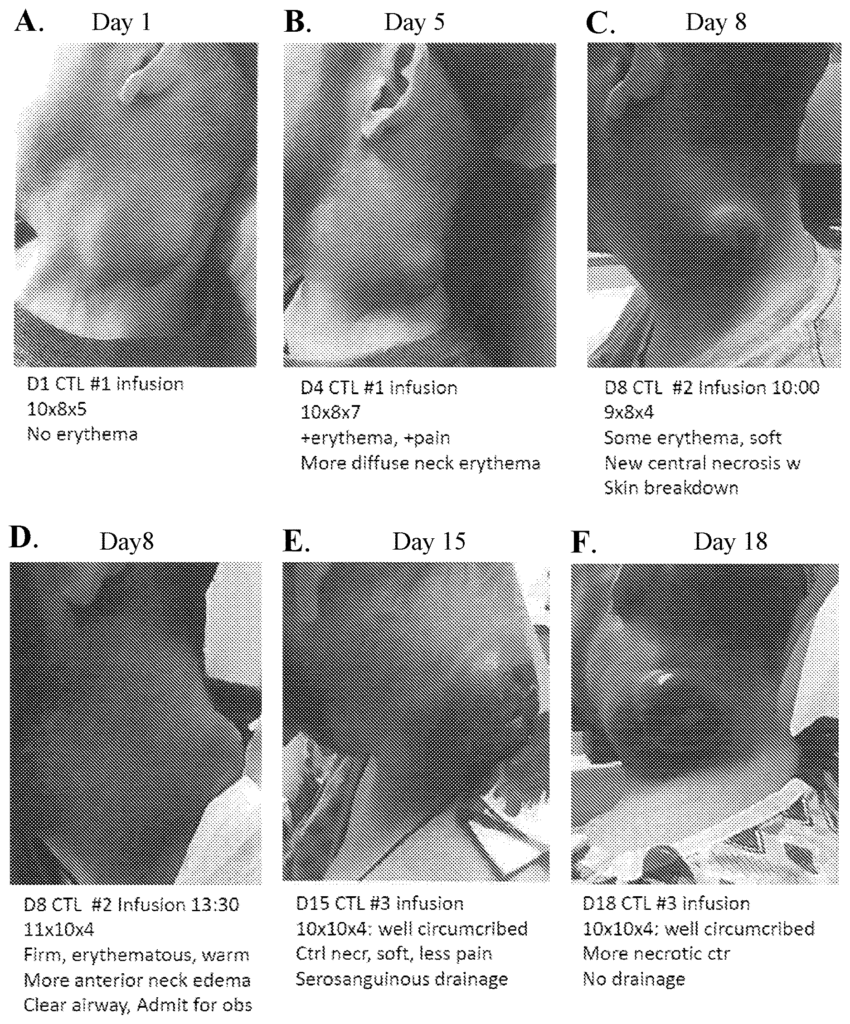
Tumor flare is a common complication in adoptive immunotherapy, particularly when treating patients with aggressive or advanced cancers. This response, triggered by an immune system attack on tumor cells, can lead to temporary tumor swelling, inflammation, and increased pain, causing distress and posing potential health risks for patients. Managing this response is crucial for healthcare providers, as it impacts treatment continuity, patient comfort, and the overall success of immunotherapy. Without effective management tools, tumor flare can undermine patient confidence and the potential of immunotherapy as a safe cancer treatment option.
Precise Tumor Flare Control for Optimal Patient Care
This tumor flare management method provides a structured approach to control and mitigate immune responses associated with adoptive immunotherapy. By incorporating specific interventions that regulate the immune response and prevent excessive inflammation, this technology minimizes the severity of tumor flare, allowing patients to continue their therapy with greater comfort and fewer interruptions. The protocol is designed to balance immune activity, ensuring that patients receive effective cancer treatment while minimizing the side effects associated with rapid tumor response. This focus on patient-centered care supports both short-term comfort and long-term therapeutic success.
Key Benefits for Oncology and Immunotherapy Providers
For pharmaceutical companies and immunotherapy providers, this technology offers a vital solution to enhance the patient experience during cancer treatment. It enables healthcare providers to implement a safer, more predictable approach to adoptive immunotherapy, increasing the therapy’s accessibility for a broader patient population. Oncology centers and immunotherapy facilities can incorporate this tumor flare management technology into their care protocols, improving treatment adherence and patient satisfaction. Its emphasis on comfort and safety aligns with the trend toward personalized medicine, making it a valuable addition to advanced cancer care practices.
Invest in Safer, Patient-Centered Cancer Treatments
Licensing this tumor flare management technology positions your company as a leader in patient-focused cancer care. By offering a sophisticated, effective solution for managing tumor flare, your business can support the future of immunotherapy and help more patients access innovative treatments with confidence. This technology is an essential investment for companies committed to improving patient outcomes, advancing immunotherapy, and supporting holistic, compassionate cancer treatment approaches.
- Abstract
- Claims
What is claimed is:
Share
Title
Methods of managing tumor flare in adoptive immunotherapy
Inventor(s)
Robert Baiocchi
Assignee(s)
Atara Biotherapeutics Inc
Patent #
11925663
Patent Date
March 12, 2024