Innovative Therapeutic Approach for Viral Diseases and Cancer Treatment
Introduction
Treating viral diseases and proliferative disorders like cancer remains one of the most significant challenges in modern medicine. While existing therapies can be effective, they often face limitations such as resistance, side effects, or inefficiencies in targeting abnormal cell growth. Our patented method offers a groundbreaking approach to treating these conditions by modulating biological pathways to combat both viral infections and uncontrolled cell proliferation, opening new possibilities for more effective and targeted treatments. This technology is poised to become a key tool for pharmaceutical companies looking to expand their therapeutic portfolios in the areas of virology and oncology.
The Complexities of Treating Viral Diseases and Proliferative Disorders
In the fight against viral diseases, traditional treatments like antiviral drugs can struggle with resistance as viruses mutate and evade therapeutic interventions. Moreover, the treatment of proliferative disorders, such as cancers, is often complicated by the difficulty in targeting abnormal cells without harming healthy tissues. This makes it imperative for new therapies to provide a more specific mechanism of action, with reduced side effects and improved patient outcomes.
For pharmaceutical companies, there is a growing need for innovative therapies that can both address viral infections and inhibit the proliferation of abnormal cells. Developing such treatments requires a deep understanding of the underlying mechanisms that drive these conditions, enabling more effective intervention at the molecular level.
A Dual-Action Approach to Treatment
Our patented method offers a unique, dual-action therapeutic approach by targeting the pathways involved in both viral replication and cellular proliferation. By modulating specific biological mechanisms, this method interrupts the processes that allow viruses to replicate within the body and simultaneously inhibits the abnormal growth of cells in proliferative disorders like cancer. This dual focus offers a powerful and versatile tool for treating a wide range of conditions, from viral infections like hepatitis and HIV to cancers of various origins.
This method is particularly valuable because it can be applied to develop treatments that are both more targeted and less likely to result in resistance. By focusing on fundamental biological processes shared by both viral diseases and proliferative disorders, this technology provides a broad platform for therapeutic development, allowing pharmaceutical companies to create drugs that can address multiple conditions with a single mechanism of action.
Key Benefits
- Dual Therapeutic Focus: Targets both viral infections and abnormal cell proliferation, offering broad therapeutic applications.
- Reduced Risk of Resistance: Provides a more specific mechanism of action, reducing the likelihood of drug resistance in viral diseases.
- Broad Applications: Suitable for treating various viral diseases and cancers, offering a versatile platform for pharmaceutical development.
- Improved Patient Outcomes: Offers a targeted approach that reduces side effects and improves efficacy compared to traditional treatments.
A Promising Platform for Future Therapies
Licensing this method for treating viral diseases and proliferative disorders gives pharmaceutical and biotechnology companies a valuable tool for expanding their research and development pipelines. With its dual-action mechanism and broad applicability, this technology opens new doors for creating highly effective treatments that address some of the most pressing challenges in modern medicine.
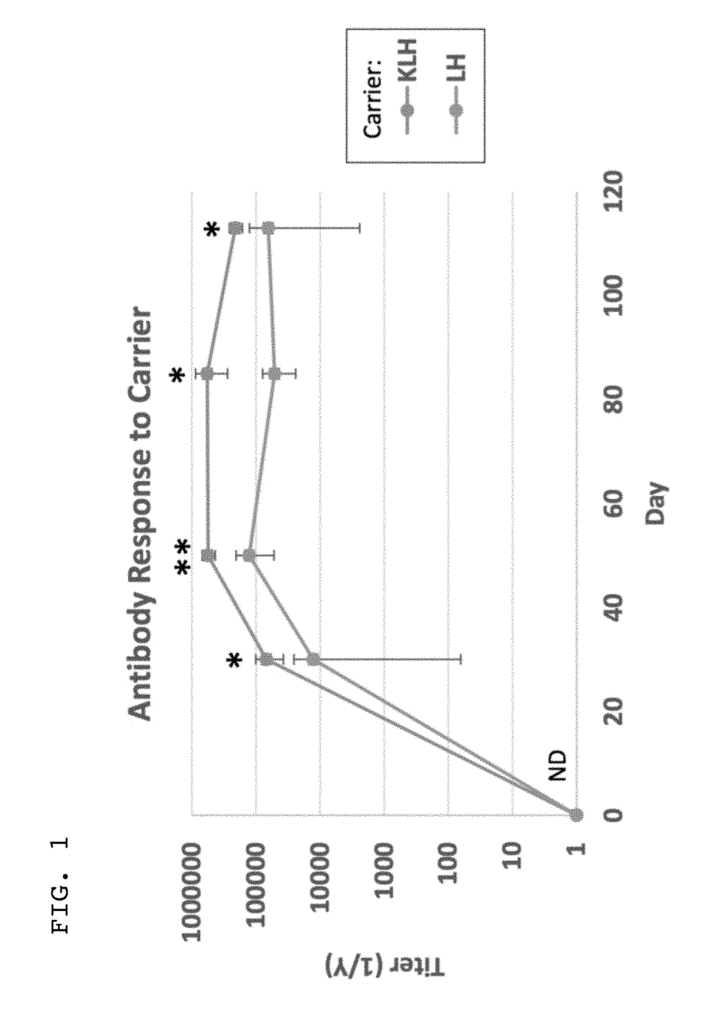
- Abstract
- Claims
What is claimed is:
Share
Title
Method of treating viral diseases and proliferative disorders
Inventor(s)
Robert C. Bayer
Assignee(s)
Lobster Unlimited LLC
Patent #
10456427
Patent Date
October 29, 2019