A New Era in Cancer Treatment: Targeted Solutions for Invasive Cancers
Introduction
Invasive cancers pose one of the most formidable challenges in modern medicine. Traditional treatments often involve aggressive therapies that target not only cancer cells but healthy tissue as well, leading to debilitating side effects. Our breakthrough composition and method offer a more refined approach—one that targets the cancer while minimizing damage to surrounding healthy cells.
The Challenge
Treating invasive cancers effectively without causing excessive harm to the patient has long been the goal of oncology. Current treatments like chemotherapy and radiation often come with severe side effects, as they cannot differentiate between cancerous and healthy cells. What’s needed is a more precise solution that aggressively combats cancer while minimizing collateral damage.
The Solution
Our innovative approach centers on a composition that specifically targets invasive cancer cells, disrupting their growth and survival mechanisms. This targeted therapy is designed to hone in on cancerous cells with remarkable accuracy, reducing the impact on healthy tissue. By utilizing this method, patients experience fewer side effects while receiving potent cancer-fighting treatment.
Why This Matters
- Precision Targeting: This method offers a highly targeted approach to cancer treatment, focusing on invasive cancer cells while sparing healthy ones. This precision helps reduce side effects, improving patient outcomes and quality of life during treatment.
- Versatile Application: The composition is adaptable for various types of invasive cancers, making it a versatile addition to any oncologist’s arsenal. Whether dealing with aggressive forms of lung, breast, or gastrointestinal cancers, this method provides a focused treatment option.
- Potential for Combination Therapies: This innovation doesn’t just stand alone—it can be integrated into existing treatment regimens, working synergistically with chemotherapy, radiation, or immunotherapy. This flexibility allows oncologists to customize treatment plans for maximum efficacy.
Why License This Technology?
By licensing this technology, you are embracing the future of oncology. This breakthrough offers your company the ability to deliver cutting-edge, targeted cancer therapies that will not only improve patient outcomes but also reduce the side effects that often accompany cancer treatments. Offering a solution that is both effective and patient-friendly can set you apart in the competitive field of cancer treatment.
The Opportunity
The fight against invasive cancer is one of the most urgent battles in healthcare. By licensing this composition and method, you can be part of the next generation of cancer treatment, offering hope and improved care to millions of patients. Step into the future of oncology and help redefine how invasive cancers are treated, with a focus on precision, safety, and efficacy.
- Abstract
- Claims
The invention claimed is:
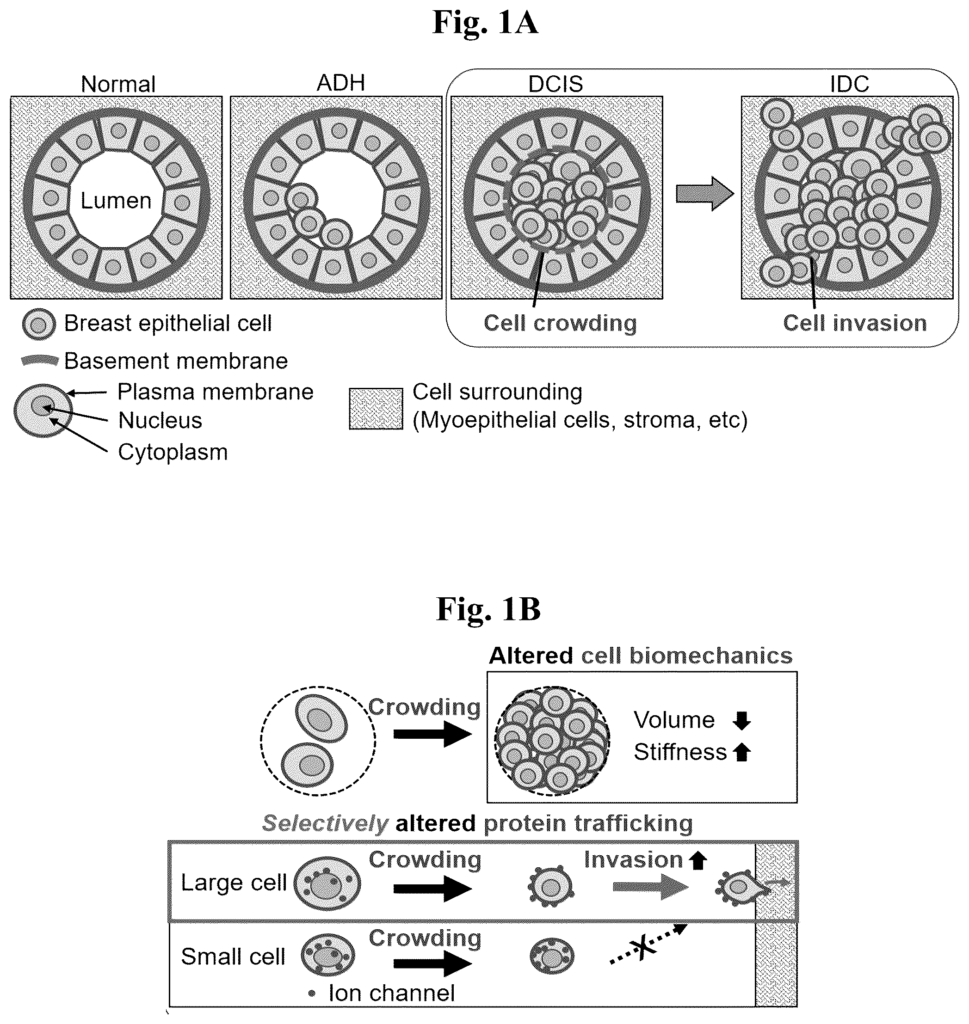
1. A method of detecting ductal carcinoma in situ (DCIS) at risk of disease progression in a subject, the method comprising:
5. The method of claim 1, further comprising calculating an invasiveness risk score
16. A method of treating a subject having ductal carcinoma in situ (DCIS) at risk of disease progression, the method comprising:
18. The method according to claim 16, wherein identifying the DCIS as having a high likelihood of becoming an invasive DCIS or an invasive breast cancer comprises
Share
Title
Compositions and methods for treatment of invasive cancers
Inventor(s)
Inhee Chung
Assignee(s)
George Washington University
Patent #
12013398
Patent Date
June 18, 2024
