A New Era of Antiviral Therapies with Self-Assembling Prodrugs
Introduction
Antiviral therapies are critical tools in the global fight against infectious diseases, especially with the rapid evolution of viral strains that threaten public health. The need for more effective antiviral treatments is growing, particularly in light of ongoing pandemics and the resurgence of viral infections. Current antiviral drugs, while effective, often face challenges related to drug resistance, low bioavailability, and off-target side effects. Our patented self-assembling antiviral prodrugs present an exciting opportunity to address these challenges with a novel approach to antiviral treatment.
Current Gaps in Antiviral Medications
Many antiviral drugs face significant barriers when it comes to delivering their active components efficiently within the body. Poor bioavailability, short half-lives, and the potential for drug resistance limit the effectiveness of conventional treatments. Additionally, antiviral therapies often require high dosages to reach therapeutic levels in target cells, which can lead to unwanted side effects and reduced patient compliance. The industry is in need of innovative drug delivery systems that ensure the right dosage reaches the right target while minimizing adverse reactions.
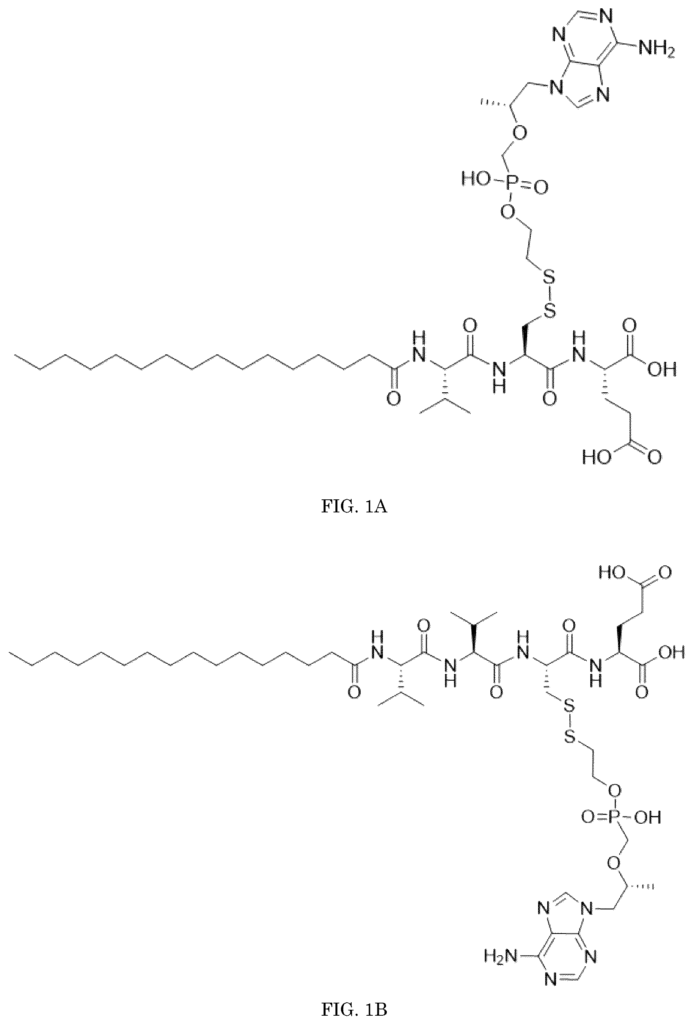
A Groundbreaking Prodrug Approach
Our self-assembling antiviral prodrugs introduce a new method for delivering antiviral agents with increased precision and efficiency. These prodrugs self-assemble at the target site, ensuring that the active antiviral compound is released directly where it is needed most. This targeted delivery minimizes the likelihood of side effects and allows for lower dosages, enhancing patient compliance and treatment outcomes.
By assembling at the site of infection, these prodrugs avoid the systemic issues typically associated with conventional antiviral therapies. They not only improve bioavailability but also reduce the risk of viral resistance by maintaining a consistent therapeutic level in the body. This technology represents a significant advance in antiviral treatment, offering a more effective, patient-friendly solution to combat viral infections.
Why These Prodrugs Stand Out
- Targeted Drug Delivery: Self-assembling prodrugs ensure the active antiviral compound is delivered directly to the infection site, maximizing effectiveness while minimizing side effects.
- Enhanced Patient Compliance: By reducing the dosage required for therapeutic effects, patients can benefit from a more tolerable treatment regimen.
- Reduced Risk of Resistance: By maintaining consistent drug levels at the infection site, this approach lowers the chance of viral mutations that lead to resistance.
- Broad Therapeutic Application: This technology has the potential to treat a wide range of viral infections, from common viral strains to emerging global health threats.
Why This Technology is a Must for Antiviral Development
Licensing this self-assembling antiviral prodrug technology positions your company at the forefront of innovative antiviral therapies. With the potential to transform how viral infections are treated, this technology offers a smarter, more efficient way to combat some of the world’s most challenging diseases, making it a critical asset for the future of pharmaceutical development.
- Abstract
- Claims
That which is claimed:
Share
Title
Self-assembling antiviral prodrugs
Inventor(s)
Honggang Cui, Charles Williams Flexner, Maya Monroe, Han Wang
Assignee(s)
Johns Hopkins University
Patent #
11998609
Patent Date
June 4, 2024





