A Targeted Solution for Mitochondrial Diseases: Hope and Innovation for Rare Genetic Disorders
Introduction
Mitochondrial diseases represent some of the most challenging medical conditions, affecting patients on a cellular level and causing severe, often life-altering symptoms. Current treatment options are limited, leaving patients and healthcare providers without effective long-term solutions. Our innovative treatment offers a breakthrough in addressing mitochondrial dysfunction, providing a targeted approach that brings real hope to individuals and families affected by these disorders.
The Problem
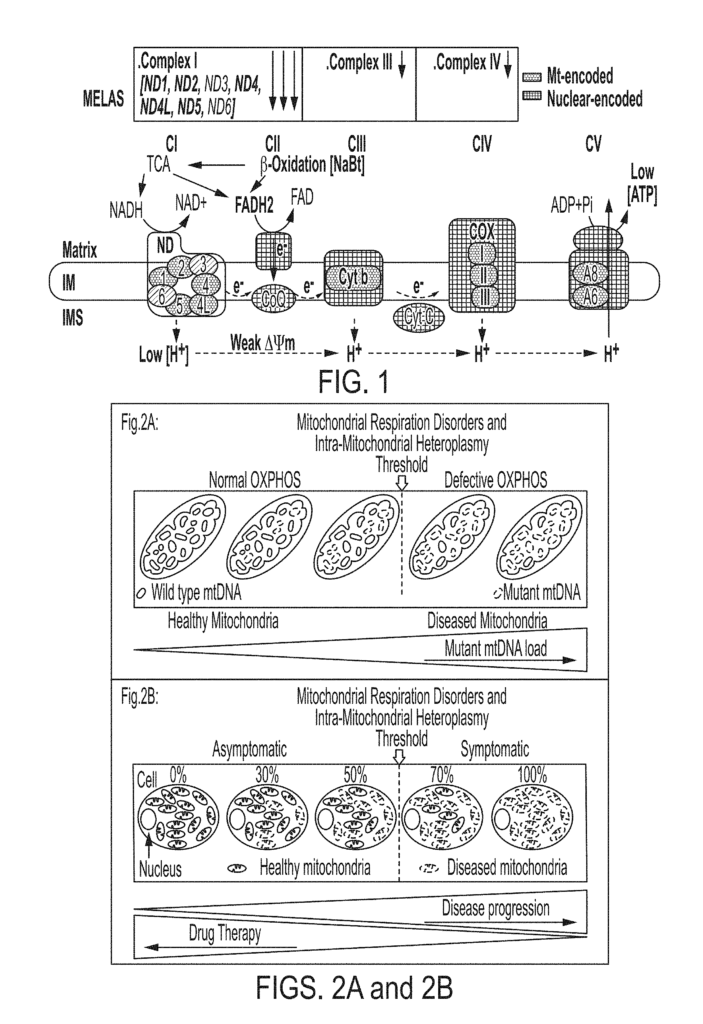
Mitochondrial diseases occur when mitochondria, the “powerhouses” of the cell, fail to function properly. These disorders can manifest in a wide range of symptoms, from muscle weakness and neurological problems to multi-system organ failure. Due to the complexity of mitochondrial function and the genetic variability of these diseases, traditional treatments have been largely ineffective in providing lasting relief or addressing the root cause of the problem.
The Solution
Our patented treatment represents a groundbreaking advancement in mitochondrial disease therapy. By specifically targeting dysfunctional mitochondria, this therapy works at the cellular level to improve mitochondrial performance, reduce symptoms, and slow disease progression. It goes beyond symptom management, aiming to address the underlying dysfunction in the mitochondria itself.
Why This Technology Stands Out
- Precision Treatment: The therapy is designed to directly target the mitochondria, providing a focused approach that enhances its ability to generate energy and maintain cellular health. This precision sets it apart from generalized treatments that fail to address the core issue.
- Wide-Ranging Impact: Mitochondrial diseases affect multiple systems in the body. By improving mitochondrial function, this treatment has the potential to alleviate symptoms across a broad range of organ systems, improving quality of life for patients with complex, multi-system disorders.
- Innovative Mechanism of Action: This treatment utilizes novel mechanisms to enhance mitochondrial function, opening up new pathways for future research and combination therapies.
The Opportunity
Licensing this technology offers pharmaceutical companies and research institutions a unique opportunity to lead in a largely underserved market. With a growing understanding of mitochondrial diseases and the need for effective treatments, this innovation positions your company to be at the forefront of genetic and mitochondrial therapy.
This is more than a treatment—it’s a transformative approach that brings real hope to a patient population in desperate need of solutions.
- Abstract
- Claims
We claim:
1. A method of treating a subject with a disease or disorder associated with a mitochondrial deficit, comprising:
Share
Title
Treatment for mitochondrial diseases
Inventor(s)
Anne Chiaramello, Martine Uittenbogaard
Assignee(s)
George Washington University
Patent #
10272056
Patent Date
April 30, 2019
