Advanced Molecular Imaging with Raman Cluster Tags for Precise Biological Insight
Introduction
In the field of medical diagnostics and biotechnology research, the ability to visualize and track biological molecules at the cellular level is critical for understanding disease mechanisms and developing new therapies. Conventional imaging methods, while effective, often face challenges in resolution, sensitivity, and specificity. Our patented Raman cluster tagged molecules offer a groundbreaking solution for biological imaging, providing a highly sensitive and precise approach to visualize molecular interactions within complex biological environments.
Limitations of Traditional Imaging Techniques
Traditional imaging methods, such as fluorescence or magnetic resonance imaging (MRI), are widely used in both research and clinical settings, but they come with certain limitations. Fluorescence imaging, for example, can suffer from photobleaching, low resolution, and interference from background signals, which affects its sensitivity and accuracy. MRI, while useful for anatomical imaging, may lack the molecular specificity needed for detecting subtle biological changes at the cellular level.
In the context of molecular biology, researchers need a method that can accurately track biological processes and molecular interactions in real-time, without interference from noise or degradation. This need is particularly pressing in areas such as cancer research, drug development, and genomics, where detecting changes at the molecular level is key to advancing science and improving patient outcomes.
A New Dimension of Precision with Raman Cluster Tags
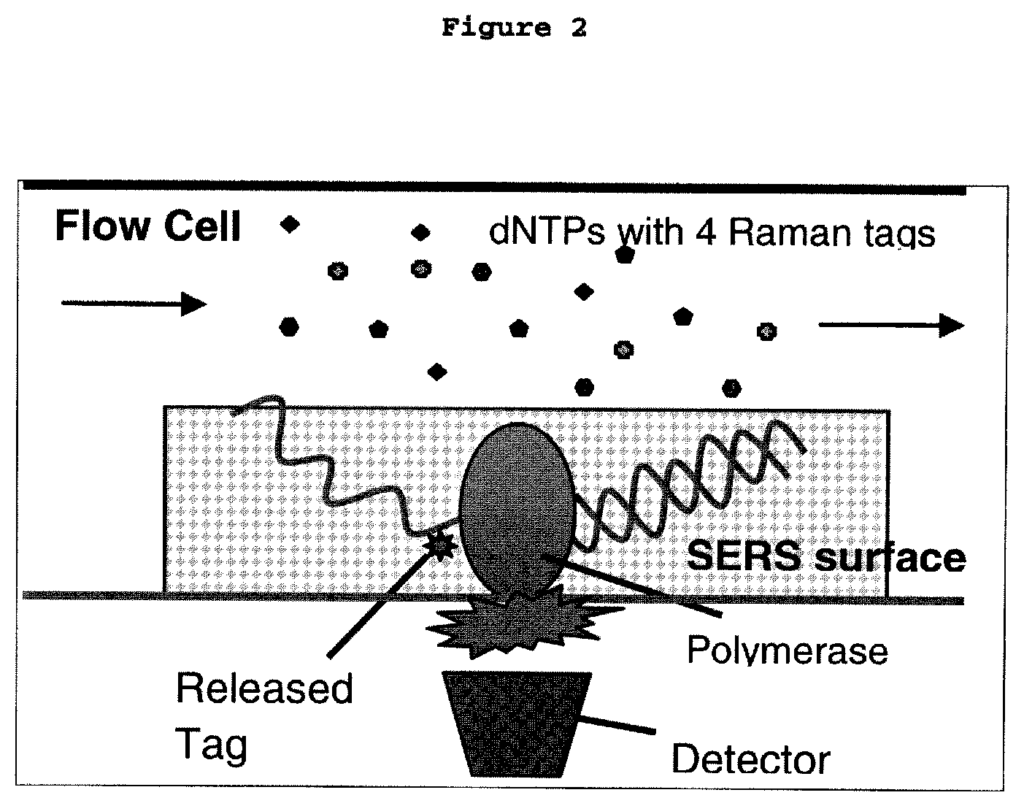
Our patented Raman cluster tagged molecules introduce a new standard in molecular imaging, offering unparalleled sensitivity and specificity for detecting and visualizing target molecules. These cluster-tagged molecules take advantage of Raman scattering, a phenomenon that provides highly specific vibrational information about molecules. Unlike traditional imaging techniques, Raman imaging is not affected by photobleaching, and the signal remains strong and stable over time.
The use of these tagged molecules enables researchers and clinicians to track molecular interactions with incredible accuracy, even within complex biological systems. This opens up a wide range of applications, from early cancer detection to monitoring drug efficacy in real-time. Moreover, the ability to detect multiple signals simultaneously makes this technology ideal for multiplexing applications, where various biomarkers need to be monitored in parallel.
Advantages of Raman Cluster Tagged Molecules
- Enhanced Sensitivity and Specificity: Raman scattering provides highly detailed molecular information, allowing for accurate detection of target molecules.
- Stable and Durable: Unlike fluorescence imaging, Raman tags do not suffer from photobleaching, ensuring stable, long-lasting signals.
- Broad Applications: From cancer diagnostics to drug discovery, this technology can be applied across various fields in medical research and biotechnology.
- Multiplexing Capability: The ability to detect multiple molecules simultaneously makes it a powerful tool for complex biological studies.
A Game-Changer in Molecular Imaging
Licensing this Raman cluster tagged molecule technology offers an opportunity to lead in advanced molecular imaging, providing researchers and clinicians with a tool that expands the boundaries of what is possible in biological research and diagnostics. With its superior sensitivity, durability, and versatility, this technology represents a critical advancement in the precision and accuracy of biological imaging.
- Abstract
- Claims
What is claimed is:
13. A method for determining the sequence of a single-stranded DNA comprising:
14. A method for determining the sequence of a single-stranded RNA comprising:
Share
Title
Raman cluster tagged molecules for biological imaging
Inventor(s)
Jingyue Ju, Shiv Kumar, Mirkó PALLA, James J. Russo
Assignee(s)
Columbia University in the City of New York
Patent #
10648026
Patent Date
May 12, 2020





