Secure and Efficient Pharmaceutical Waste Disposal for Health and Safety
Introduction
Pharmaceutical waste disposal is a critical issue across healthcare facilities, pharmacies, and manufacturing plants. The improper disposal of unused or expired drugs can lead to environmental contamination, legal repercussions, and compromised safety. In response to the growing need for compliant, secure, and efficient disposal systems, our pharmaceutical waste disposal assembly offers a unique solution that ensures safe handling, separation, and disposal of pharmaceutical waste, protecting both public health and the environment.
The Challenge
Improper disposal of pharmaceutical waste can result in contamination of water sources, ecosystems, and even human populations. Regulatory agencies impose strict guidelines on how pharmaceutical waste must be managed, and healthcare facilities and manufacturers are under increasing pressure to comply. However, current waste disposal systems often lack the necessary mechanisms to divert hazardous materials efficiently, leading to costly mistakes and safety risks. This situation requires a more streamlined, secure, and compliant method of waste handling.
The Solution
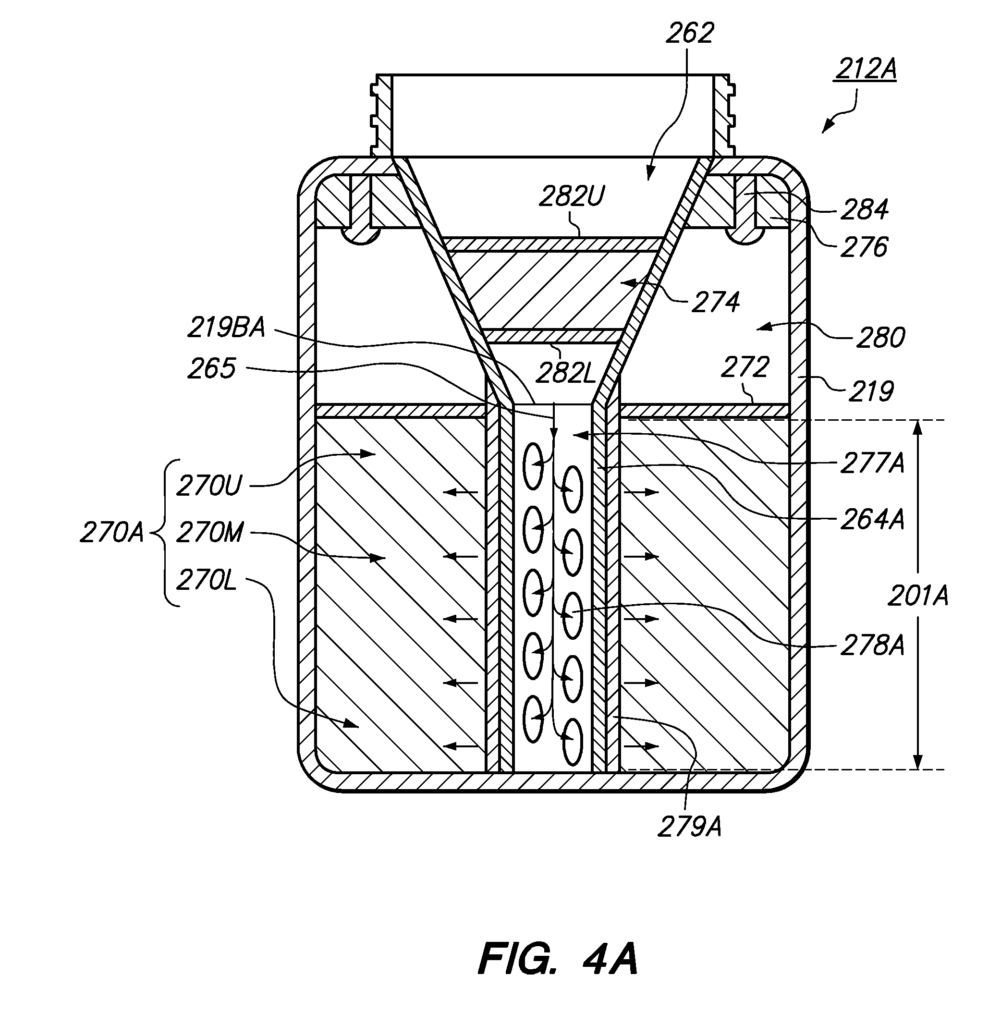
Our patented pharmaceutical waste disposal assembly addresses these challenges through a sophisticated system that incorporates a waste diverter. This assembly enables the secure separation and disposal of different types of pharmaceutical waste—whether it’s hazardous, non-hazardous, or bioactive—ensuring compliance with all regulatory requirements.
The waste diverter plays a key role in streamlining the process, efficiently diverting materials into the appropriate containers, reducing the risk of cross-contamination. With clear labeling and an easy-to-use design, this assembly is ideal for hospitals, pharmacies, and pharmaceutical manufacturers seeking an efficient, secure, and compliant waste management system.
Key Benefits
- Regulatory Compliance: The assembly is designed to meet stringent disposal regulations, ensuring that healthcare facilities and manufacturers remain compliant with all environmental and safety guidelines. It mitigates risks of fines or legal issues from improper waste disposal.
- Enhanced Safety: By preventing cross-contamination and securely handling hazardous waste, this system protects staff, patients, and the environment from the harmful effects of pharmaceutical contamination.
- Efficiency in Waste Diversion: The waste diverter ensures that different types of pharmaceutical waste are separated and disposed of properly, streamlining the process and reducing operational downtime. It simplifies waste management for healthcare providers, reducing errors and improving workflow.
- Environmentally Responsible: By managing waste responsibly, this system helps reduce the environmental impact of improperly disposed drugs, keeping waterways and ecosystems free from pharmaceutical pollutants.
Why License This Technology?
Licensing this pharmaceutical waste disposal assembly offers hospitals, pharmacies, and manufacturers an effective and reliable solution to a growing problem. By integrating this system, your organization can ensure regulatory compliance, enhance workplace safety, and contribute to a cleaner, safer environment. This technology not only solves a pressing operational challenge but also demonstrates a commitment to health and environmental responsibility.
- Abstract
- Claims
What is claimed is:
Share
Title
Pharmaceutical waste disposal assembly including waste diverter
Inventor(s)
David A. Maness
Assignee(s)
Stryker Corp
Patent #
8616397
Patent Date
December 31, 2013
