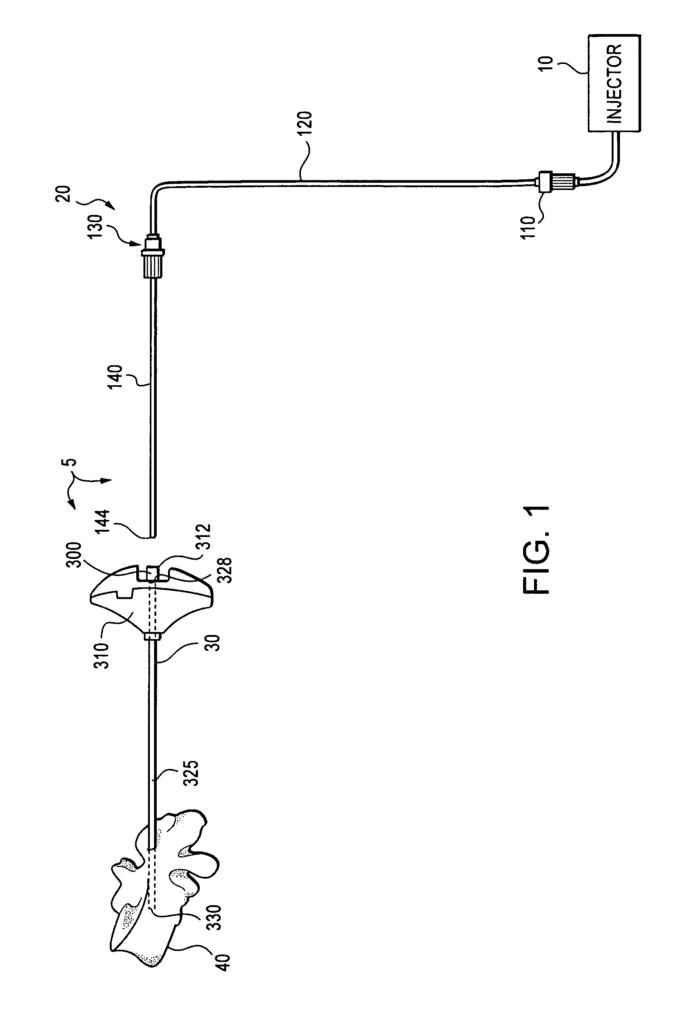
Delivering curable materials in precise amounts and at the right moment is essential in numerous fields, from medical procedures to industrial applications. Whether it’s delivering bone cement in orthopedic surgery or applying adhesives in a manufacturing setting, accuracy and efficiency can be the difference between success and costly errors. This curable material delivery device offers an advanced solution for professionals needing precise control over material flow in time-sensitive situations.
The Challenge
In both medical and industrial settings, delivering curable materials like bone cement, dental composites, or construction adhesives can be fraught with challenges. Improper mixing, inconsistent flow, or timing issues can lead to compromised outcomes. For example, in surgery, improper delivery of bone cement could result in joint instability or increased patient recovery time. Similarly, in construction or dental applications, inaccurate application of adhesives can lead to structural weakness or product failure.
The demand for a device that simplifies material handling while ensuring accuracy and consistency is growing. The complexity of current tools often results in delays or errors that negatively affect performance, adding cost and risk to the procedure or process.
The Solution
Our patented curable material delivery device is designed to address these challenges head-on. By offering a system that allows precise control over the flow and timing of curable materials, it ensures consistent application in critical procedures. This tool not only streamlines operations but also enhances precision, reducing waste and improving overall outcomes.
With an ergonomic design, this device is easy to handle and operate, ensuring that users across industries can deliver curable materials in an accurate, timely manner. Its adaptable design allows it to work with various materials—bone cement in medical applications, dental restoratives, or even industrial sealants.
Key Benefits
- Precision and Control: This device allows for exact delivery, ensuring materials are applied consistently and accurately, reducing the chance of error in time-sensitive situations.
- Versatile Applications: From orthopedic surgeries to dental restorations and industrial settings, this delivery device works with a range of curable materials and settings.
- Time-Saving: The streamlined design of the device simplifies the process, reducing preparation time, and allowing professionals to focus on the task at hand, improving efficiency and reducing downtime.
- Reduced Waste: By controlling the amount of material dispensed, the device minimizes waste, contributing to cost savings and sustainability in both medical and industrial environments.
Why License This Technology?
By licensing this curable material delivery device, you are providing your team or customers with a tool that enhances both accuracy and efficiency. The ability to deliver materials precisely and consistently is crucial in high-stakes environments, whether in medical, dental, or industrial applications. This technology simplifies complex processes, reduces risk, and improves outcomes, making it an invaluable addition to any practice or industry seeking improved performance and reliability.