Targeted Solutions for Treating Drug-Resistant Bacterial Infections
Introduction
The rise of antibiotic-resistant bacteria represents one of the most significant challenges in modern medicine. Traditional antibiotics, while once highly effective, are increasingly limited in their ability to combat persistent and drug-resistant bacterial strains such as Mycobacterium tuberculosis. With bacterial infections becoming harder to treat, there is a growing need for innovative antibiotic solutions that address these challenges head-on. Our patented oxazolidinones offer an advanced, targeted approach to treating bacterial infections, providing healthcare professionals and pharmaceutical companies with a much-needed tool in the fight against antibiotic resistance.
Why Current Antibiotics Are Losing Ground
As bacteria evolve and develop resistance to commonly used antibiotics, traditional treatments are becoming less effective, leading to prolonged illness, increased mortality, and the spread of untreatable infections. This is particularly concerning with pathogens like Mycobacterium tuberculosis, a major global health issue. Patients often face extended treatments with toxic drug regimens that may not even guarantee success. The development of new antibiotics with novel mechanisms of action is critical to overcoming these obstacles and addressing the global health crisis of drug-resistant bacterial infections.
A Promising New Class of Antibiotics
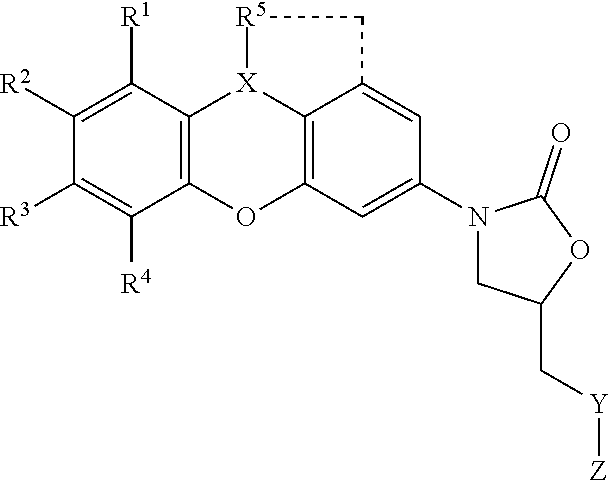
Our patented oxazolidinones represent a cutting-edge approach to treating drug-resistant bacterial infections, offering a unique mechanism of action that targets bacteria at the cellular level. This antibiotic class is particularly effective against Mycobacterium tuberculosis, the bacterium responsible for tuberculosis (TB). By inhibiting bacterial protein synthesis, oxazolidinones are able to effectively kill the pathogen, even in strains that have developed resistance to other antibiotics.
These compounds also demonstrate broad-spectrum efficacy, meaning they can be utilized to treat a wide range of bacterial infections beyond TB. This versatility makes oxazolidinones an attractive option for pharmaceutical companies looking to expand their antibiotic portfolios and address the global need for more effective bacterial infection treatments.
What Sets This Technology Apart
- Targeted Mechanism: The oxazolidinones’ ability to inhibit protein synthesis in bacteria provides a powerful new tool for treating drug-resistant infections.
- Effective Against TB: Specifically designed to combat Mycobacterium tuberculosis, this technology offers a much-needed solution to the rising threat of drug-resistant TB.
- Broad-Spectrum Application: Beyond TB, oxazolidinones can be used to treat a variety of bacterial infections, making it a versatile and valuable addition to any antibiotic arsenal.
- Addressing Drug Resistance: These compounds represent a critical advancement in antibiotic development, targeting resistant strains that have outpaced current treatments.
An Essential Tool for Modern Antibiotic Therapy
Licensing this oxazolidinone-based antibiotic technology provides a critical opportunity for pharmaceutical companies to lead in the development of life-saving treatments for drug-resistant bacterial infections. With the growing global threat of antibiotic resistance, this technology offers a solution that is not only effective but also adaptable for a range of bacterial diseases, making it a vital asset for future healthcare innovations.
- Abstract
- Claims
What is claimed is:
Share
Title
Oxazolidinones and pharmaceutical compositions thereof for treating bacterial infections, including infection of Mycobacterium tuberculosis
Inventor(s)
J. Thomas Ippoliti, Gyanu Lamichhane
Assignee(s)
Johns Hopkins University, University of St Thomas
Patent #
10870646
Patent Date
December 22, 2020








