Innovative and Life-Changing Activin Receptor Therapy for Healing
Introduction
This stabilized activin IIB receptor polypeptide technology offers a groundbreaking approach to enhancing muscle growth, repair, and regeneration, presenting new possibilities for treating muscular disorders and supporting tissue recovery. Designed to remain stable and active in various biological conditions, this polypeptide therapy provides a targeted method to promote muscle strength, mass, and repair, making it invaluable for patients facing muscle-wasting diseases, recovery challenges, or other regenerative health needs. For companies in pharmaceuticals and regenerative medicine, this activin receptor technology represents a unique opportunity to develop treatments that transform patient care and elevate therapeutic outcomes.
The Challenge: Managing Muscle-Wasting and Regenerative Needs
Muscle-wasting diseases, such as muscular dystrophy and sarcopenia, pose a significant health challenge, affecting millions and often leaving patients with limited options for effective treatment. These conditions result in muscle degeneration, weakness, and a diminished quality of life, with few existing therapies targeting the underlying biological factors that govern muscle regeneration and strength. Healthcare providers and patients are in urgent need of solutions that can address these needs and support better recovery and muscle preservation, particularly as demand grows for regenerative and muscle-supportive therapies.
Stabilized Activin IIB for Enhanced Muscle Health
This activin receptor therapy technology leverages stabilized polypeptides to activate critical pathways involved in muscle growth and repair. By enhancing the activin IIB receptor’s stability, this technology ensures consistent therapeutic effects, even in challenging biological environments, leading to more effective treatment outcomes. Patients benefit from targeted muscle recovery, improved strength, and long-term muscle preservation, making this therapy ideal for those with muscular degenerative diseases, post-surgical recovery needs, or regenerative therapy requirements. This unique mechanism addresses the core biological processes in muscle health, offering a tailored approach to healing and recovery.
Key Benefits for Pharmaceuticals and Regenerative Medicine
For pharmaceutical companies, this technology presents a distinct advantage in creating a line of therapeutic products that specifically target muscle growth, repair, and preservation. Healthcare providers in regenerative medicine can integrate this therapy to support patients experiencing muscle degradation, offering a reliable, scientifically advanced solution for long-term muscle health. Its targeted action on activin IIB receptors allows for applications across diverse therapeutic areas, including recovery from injury, degenerative disease support, and enhanced quality of life for aging populations.
Invest in Transformative Muscle Recovery Solutions
Licensing this activin receptor therapy positions your company as a leader in regenerative medicine and muscle health. By offering an effective, stabilized solution for muscle and tissue repair, your business can address the growing demand for advanced therapeutic solutions that improve patient outcomes and quality of life. This technology is a valuable investment for companies committed to developing innovative treatments in muscle health, recovery, and regenerative care.
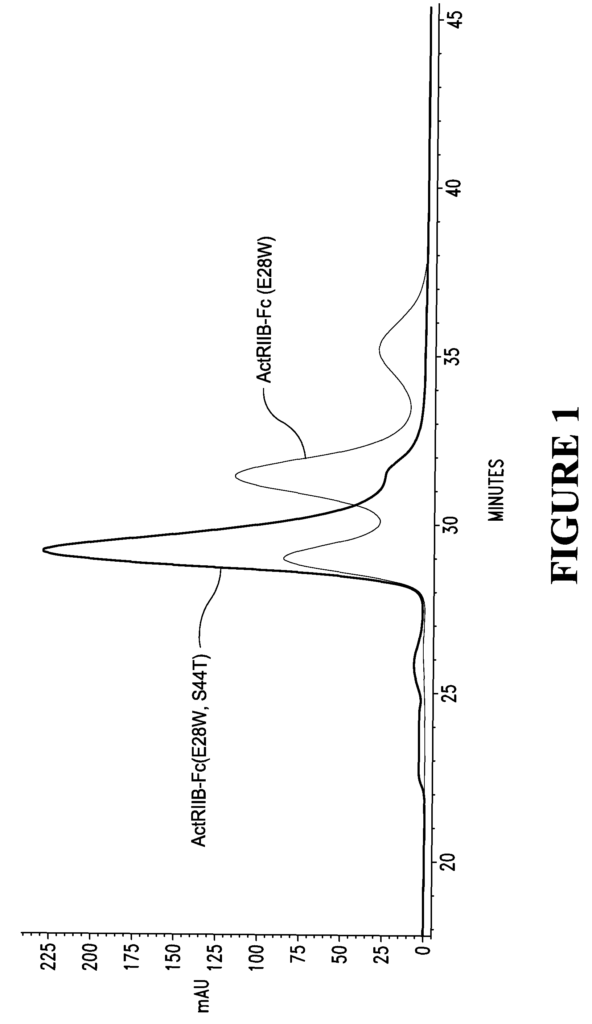
- Abstract
- Claims
What is claimed is:
1. An isolated protein comprising a stabilized activin IIB receptor polypeptide (svActRIIB) wherein said polypeptide is selected from the group consisting of:
Share
Title
Stabilized activin IIB receptor polypeptides and uses thereof
Inventor(s)
Jeonghoon Sun, Lei-Ting Tony Tam, Mark Leo Michaels, Thomas C. Boone, Rohini Deshpande, Yue-Sheng LiHq Han
Assignee(s)
Amgen Inc, Atara Biotherapeutics Inc
Patent #
8410043
Patent Date
April 2, 2013
