Transform Fuel Cell Technology with Our Ultralow Platinum, High-Durability Membrane Electrode Assembly
Introduction
In the rapidly evolving field of clean energy, Polymer Electrolyte Membrane (PEM) fuel cells are at the forefront, offering efficient, zero-emission power for a range of applications, from automotive to stationary power systems. However, the widespread adoption of PEM fuel cells has been hindered by two critical challenges: the high cost of platinum catalysts and the durability of the membrane electrode assembly (MEA). Our patented “Method for Making Ultralow Platinum Loading and High Durability Membrane Electrode Assembly for Polymer Electrolyte Membrane Fuel Cells” (Patent #2020/0106107) provides a breakthrough solution to these challenges.
The Challenge
Platinum is an essential catalyst in PEM fuel cells, but its high cost significantly increases the overall expense of fuel cell production, limiting the commercial viability of this clean energy technology. Additionally, the durability of the MEA is crucial for the longevity and reliability of fuel cells. Conventional methods struggle to balance platinum loading with the durability of the assembly, often compromising one for the other.
The Solution
Our patented method revolutionizes the manufacturing of MEAs by dramatically reducing the platinum content without sacrificing performance or durability. Here’s why this technology is a game-changer and why you should consider licensing it:
-
- Significant Cost Reduction: By utilizing ultralow platinum loading, this technology drastically lowers the cost of PEM fuel cells. Reducing platinum content directly translates to lower material costs, making fuel cells more affordable and competitive with other energy solutions. This cost advantage opens new markets and applications for PEM fuel cells, making them viable for broader commercial and consumer use.
-
- Enhanced Durability: Durability is a critical factor in the commercial success of fuel cells, particularly for automotive and stationary applications where long operational lifespans are essential. Our patented method not only reduces platinum usage but also enhances the durability of the MEA. This ensures that the fuel cells can withstand rigorous operating conditions over extended periods, providing reliable and consistent performance.
-
- Improved Performance: Despite the lower platinum content, this technology maintains high catalytic activity and efficiency. The optimized structure and composition of the MEA enable efficient electrochemical reactions, ensuring that the fuel cells deliver the power output required for demanding applications.
-
- Environmental Impact: By reducing the reliance on platinum, a rare and expensive metal, this technology contributes to more sustainable fuel cell production. This aligns with global efforts to reduce reliance on scarce resources and minimize the environmental impact of clean energy technologies.
-
- Market Potential: The demand for clean and efficient energy solutions is growing rapidly across various sectors. Licensing this technology positions your company at the forefront of this growth, providing you with a competitive edge in the expanding fuel cell market. Whether for automotive, industrial, or residential applications, this technology offers a cost-effective and reliable solution that meets the increasing demand for sustainable energy.
The Opportunity
By licensing our patented method for ultralow platinum loading and high-durability MEA, you gain access to a cutting-edge technology that addresses the key challenges in PEM fuel cell production. This technology not only reduces costs but also enhances performance and durability, providing a clear pathway to commercial success in the clean energy sector.
Don’t miss the opportunity to be a leader in the next generation of fuel cell technology. License this innovation today and drive the future of clean energy with more affordable, durable, and efficient fuel cells.
- Abstract
- Claims
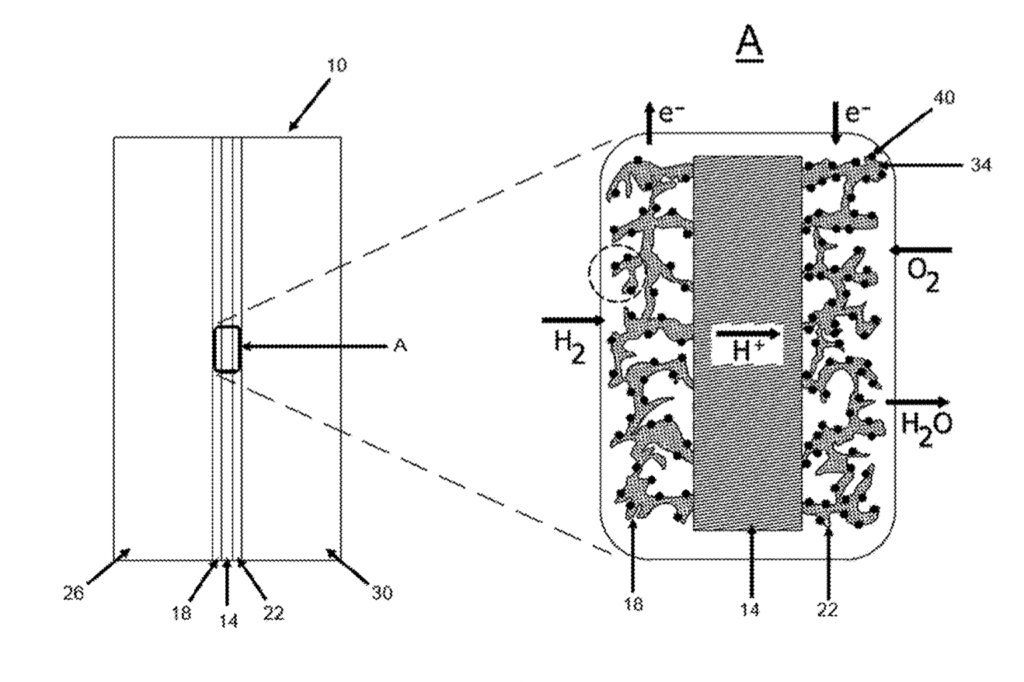
A method of making a catalyst layer of a membrane electrode assembly ( MEA ) for a polymer electrolyte membrane fuel cell includes the step of preparing a porous buckypaper layer comprising at least one selected from the group consisting of carbon nanofibers and carbon nanotubes . Plati num group metal nanoparticles are deposited in a liquid solution on an outer surface of the buckypaper to create a platinum group metal nanoparticle buckypaper . A proton conducting electrolyte is deposited on the platinum group metal nanoparticles by electrophoretic deposition to create a proton – conducting layer on the an outer surface of the platinum nanoparticles . An additional proton – conducting layer is deposited by contacting the platinum group metal nanoparticle buckypaper with a liquid proton – conducting composition a solvent . The platinum group metal nano particle buckypaper is dried to remove the solvent . A membrane electrode assembly for a polymer electrolyte membrane fuel cell is also disclosed .
We claim : 1. A method of making a catalyst layer of a membrane electrode assembly ( MEA ) for a polymer electrolyte membrane fuel cell , comprising the steps of : preparing a porous buckypaper layer comprising at least one selected from the group consisting of carbon nano fibers and carbon nanotubes ; depositing platinum group metal nanoparticles in a liquid solution on an outer surface of the buckypaper to create a platinum nanoparticle buckypaper ; and , depositing a proton conducting electrolyte on the plati num nanoparticles by electrophoretic deposition to create a proton – conducting layer on the an outer surface of the platinum nanoparticles ; depositing an additional proton – conducting layer by con tacting the platinum nanoparticle buckypaper with a liquid proton – conducting composition in a solvent ; drying the platinum nanoparticle buckypaper to remove the solvent . 2. The method of claim 1 , wherein the step of contacting the platinum nanoparticle buckypaper with a liquid proton conducting composition in a solvent comprises at least one selected from the group consisting of the liquid drop method and the liquid dipping method . 3. The method of claim 1 , wherein the proton – conducting electrolyte comprises at least one selected from the group consisting of Nafion , polyvinylidene fluoride ( PVDF ) / Nafion composite , and Nafion / silica composite . 4. The method of claim 1 , wherein the proton – conducting layer is from 2-10 wt % , based on the total weight of the catalyst layer . 5. The method of claim 1 , wherein the buckypaper has a porosity of from 50 % to 90 % before the deposition of the platinum group metal nanoparticles and the proton – conduct ing layer . 6. The method of claim 1 , wherein the buckypaper layer has a graduated porosity , with the porosity being less on a side of the buckypaper layer to abut a proton exchange membrane of the membrane electrode assembly . 7. The method of claim 6 , wherein the porosity of the buckypaper layer is graduated from a maximum porosity difference of 40 % to a minimum porosity difference of 10 % . 8. The method of claim 1 , wherein the platinum group metal nanoparticles are deposited electrochemically . 9. The method of claim 1 , wherein the platinum group metal nanoparticles have a dimension of from 2 to 10 nm . 10. The method of claim 1 , wherein the platinum group metal nanoparticles are from 30 to 80 % wt % , based on the total weight of the catalyst layer . 11. The method of claim 1 , wherein the buckypaper has less than 1 % binder , based on the total weight of the buckypaper layer . 12. The method of claim 1 , wherein the platinum group metal ( PGM ) nanoparticles comprise at least one selected from the group consisting of platinum , platinum nickel alloy , platinum copper alloy , platinum cobalt alloy , platinum iron alloy , platinum iridium alloy , and platinum palladium alloy . 13. The method of claim 1 , wherein the platinum group metal nanoparticles are core – shell structures including a platinum shell and a core comprising at least one selected from the group consisting of nickel , copper , cobalt , iron , iridium , and palladium . 14. The method of claim 1 , wherein the proton conductivity of the proton – conducting layer is from 0.01-0.2 Siemens / cm . 15. A membrane electrode assembly ( MEA ) for polymer electrolyte membrane fuel cells ( PEMFCs ) , comprising a catalyst layer , the catalyst layer comprising : a porous buckypaper layer comprising at least one selected from the group consisting of carbon nanofibers and carbon nanotubes , the buckypaper having an outer surface ; platinum group metal nanoparticles on the outer surface of the buckypaper , and having outer surfaces ; a proton – conducting electrolyte layer deposited on outer surfaces of the buckypaper and the platinum group metal nanoparticles , the proton conducting layer comprises a first , electrophoretically deposited proton – con ducting layer on the platinum group metal nanoparticles , and a second proton – conducting layer deposited by a liquid contact method on the Pt particles and the buckypaper . 16. The membrane electrode assembly of claim 15 , further comprising a proton exchange membrane . 17. The membrane electrode assembly of claim 16 , further comprising a second catalyst layer , the proton exchange membrane being positioned between the catalyst layers . 18. The membrane electrode assembly of claim 15 , wherein the proton – conducting electrolyte comprises at least one selected from the group consisting of Nation , polyvinylidene fluoride ( PVDF ) / Nafion composite , and Nafion / silica composite . 19. The membrane electrode assembly of claim 15 , wherein the proton – conducting electrolyte layer is from 2-10 wt % , based on the total weight of the catalyst layer . 20. The membrane electrode assembly of claim 15 , wherein the buckypaper has a porosity of from 50 % to 90 % before deposition of the platinum group metal nanoparticles and the proton – conducting layer . 21. The membrane electrode assembly of claim 20 , wherein the buckypaper layer has a graduated porosity , with the porosity being less on a side of the buckypaper layer abutting the proton exchange membrane of the membrane electrode assembly . 22. The membrane electrode assembly of claim 21 , wherein the porosity is graduated from a high of from a maximum porosity difference of 40 % to a minimum porosity difference of 10 % . 23. The membrane electrode assembly of claim 15 , wherein the liquid contact method comprises at least one selected from the group consisting of a liquid dropping method and a liquid dripping method . 24. The membrane electrode assembly of claim 15 , wherein the buckypaper has less than 1 % binder , based on the total weight of the buckypaper layer . 25. The membrane electrode assembly of claim 15 , wherein the platinum group metal ( PGM ) nanoparticles comprise at least one selected from the group consisting of platinum , platinum nickel alloy , platinum copper alloy , platinum cobalt alloy , platinum iron alloy , platinum iridium alloy , and platinum palladium alloy . 26. The membrane electrode assembly of claim 15 , wherein the platinum group metal nanoparticles are core shell structures including a platinum shell and a core comprising at least one selected from the group consisting of nickel , copper , cobalt , iron , iridium , and palladium . 27. The membrane electrode assembly of claim 15 , wherein the platinum group metal nanoparticles have a dimension of from 2-10 nm . 28. The membrane electrode assembly of claim 15 , wherein the platinum group metal nanoparticles are from 30 to 80 % of the catalyst layer , based on the total weight of the catalyst layer . 29. The membrane electrode assembly of claim 15 , wherein the proton conductivity is from 0.01-0.2 Siemens / cm . 30. The membrane electrode assembly of claim 15 , further comprising two electrically conductive and porous gas diffusion layers ( GDL ) connected to the surface of two catalyst layers . 31. The membrane electrode assembly of claim 15 , wherein the catalyst layer is a cathode catalyst layer .
Share
Title
METHOD FOR MAKING ULTRALOW PLATINUM LOADING AND HIGH DURABILITY MEMBRANE ELECTRODE ASSEMBLY FOR POLYMER ELECTROLYTE MEMBRANE FUEL CELLS
Inventor(s)
Jian-ping Zheng
Assignee(s)
Florida State University Research Foundation Inc
Publication #
20200106107
Publication Date
April 2, 2020
