Bioactive Dental Composites for Lasting Care
Introduction
Dental care has evolved significantly in recent years, yet traditional composites still present limitations when it comes to long-term durability and biological compatibility. Our patented bioactive dental composite offers a breakthrough in restorative dentistry, combining strength, aesthetics, and bioactivity to ensure longer-lasting restorations that promote oral health. This innovative material is designed to improve both patient outcomes and dental practice efficiency, offering a new gold standard in dental care.
Current Limitations in Dental Composites
While conventional dental composites provide an effective means for filling cavities and restoring damaged teeth, they often lack bioactivity. This means that although they fill a space, they do not actively contribute to the healing or health of the surrounding tissues. Over time, these composites may also degrade, requiring repairs or replacements that can be costly and inconvenient for both patients and practitioners.
Additionally, composites that do not bond well to the tooth structure can leave gaps where bacteria can enter, leading to further decay and complications. For the dental industry to move forward, bioactive materials that interact positively with oral tissues and enhance overall oral health are essential.
Why Choose Bioactive Dental Composites?
Our bioactive dental composite technology offers an innovative solution by combining the structural benefits of traditional composites with the bioactive properties that promote healing and remineralization. This composition actively releases beneficial ions, such as calcium and phosphate, which encourage the repair and regeneration of tooth tissue while helping to prevent further decay.
This bioactivity reduces the need for repeat dental procedures and promotes long-term patient satisfaction by strengthening the bond between the composite and the tooth. The material is also aesthetically pleasing, offering a natural appearance that blends seamlessly with the patient’s existing teeth.
For dental practitioners, this bioactive composite simplifies procedures, reducing the likelihood of recurrent issues and enhancing the quality of care provided. It’s a win for both dentists and patients, as it increases efficiency while improving oral health outcomes.
Key Benefits
- Promotes Healing: Actively releases ions that support the regeneration and remineralization of tooth tissue.
- Durable and Aesthetic: Combines long-lasting strength with a natural appearance.
- Enhanced Bonding: Provides a better bond to the tooth structure, reducing the risk of future decay.
- Patient Satisfaction: Improves outcomes and reduces the need for future dental repairs or replacements.
Empower Dental Health with Bioactive Composites
Licensing this bioactive dental composite technology offers dental practices a chance to lead with advanced materials that deliver superior results. By improving oral health, promoting healing, and ensuring long-lasting durability, these composites offer a pathway to better patient care and greater efficiency in dental procedures.
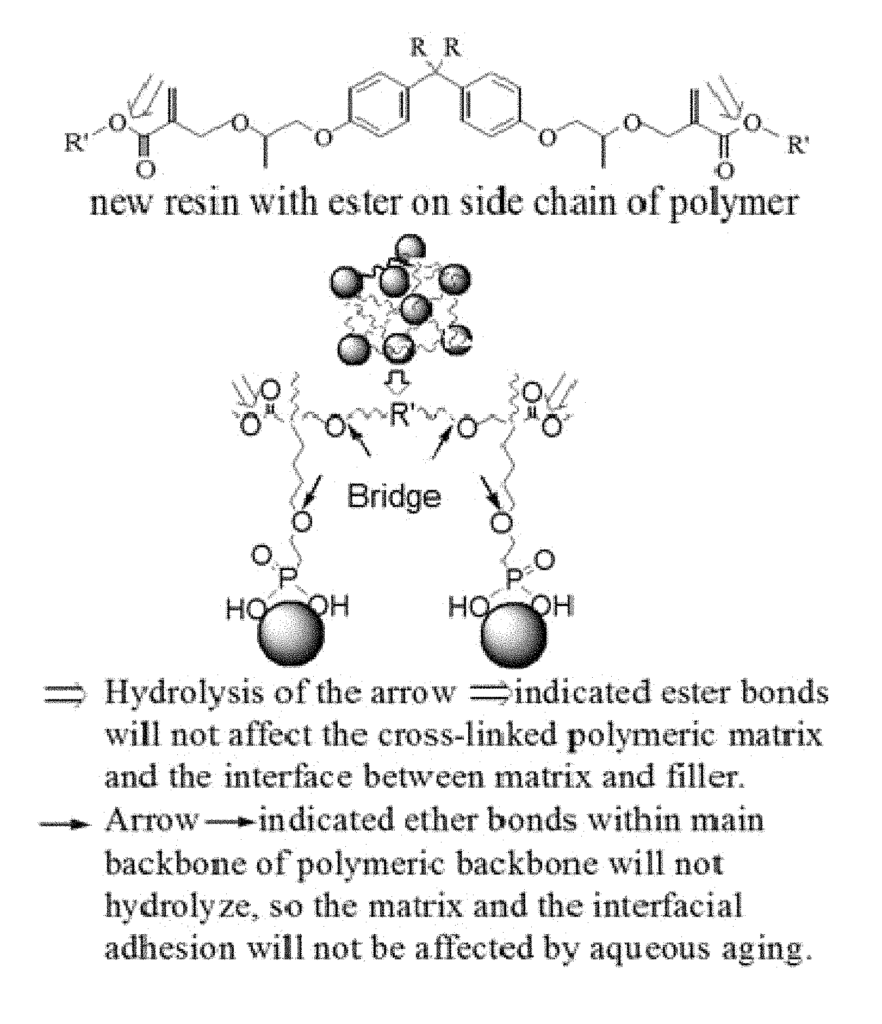
- Abstract
- Claims
What is claimed is:
1. A dental composition comprising:
5. A dental composition comprising:
15. The dental com-position of claim 1, A dental composition comprising:
Share
Title
Compositions and methods for bioactive dental composites
Inventor(s)
Tongxin Wang, Lawrence C. Chow
Assignee(s)
Howard University
Patent #
10485735
Patent Date
November 26, 2019





