Efficient Growth of Water-Soluble Magnetic Nanomaterials
Introduction
This cutting-edge method for the continuous growth of water-soluble magnetic nanomaterials offers an efficient, scalable solution for industries that require precise control over nanomaterial synthesis. Magnetic nanomaterials are widely used across multiple sectors, including medical imaging, environmental remediation, and data storage. By enabling continuous growth, this technology ensures a consistent supply of high-quality nanomaterials that can be tailored for specific applications. Companies in nanotechnology, biotechnology, and environmental science will find this innovation essential for driving new developments and expanding their capabilities.
The Challenge: Producing Water-Soluble Magnetic Nanomaterials
Magnetic nanomaterials hold immense potential across a variety of industries, but their production poses significant challenges. Traditional batch processes often result in inconsistent quality, scalability issues, and difficulty in achieving water solubility, which is critical for biological and environmental applications. As demand for these materials grows, companies need more efficient and scalable methods for producing nanomaterials that are both water-soluble and of consistent high quality. Overcoming these production challenges can unlock new applications and innovations in medical devices, environmental solutions, and beyond.
Continuous Growth for Consistent Quality
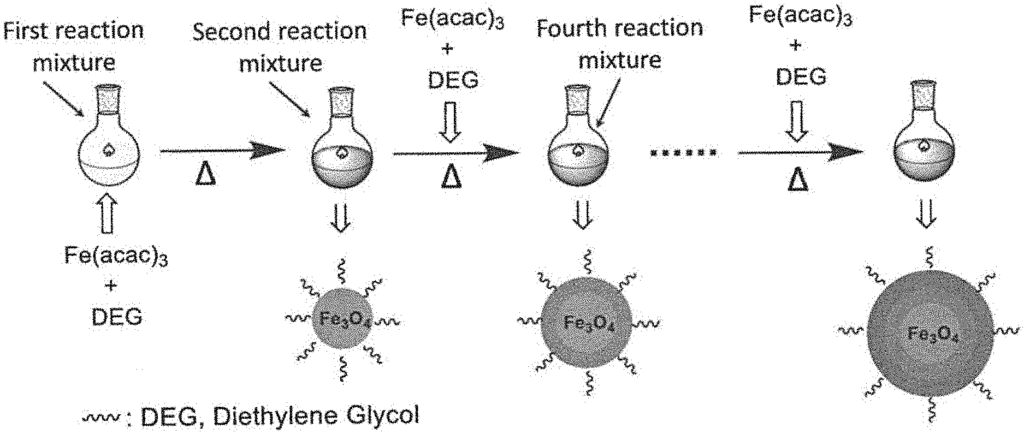
This method for the continuous growth of water-soluble magnetic nanomaterials addresses the limitations of traditional production processes. By allowing for continuous synthesis, the technology ensures consistent quality and scalability, meeting the demands of industrial and research applications. The nanomaterials produced are ideal for use in medical imaging, targeted drug delivery, and environmental remediation, as their water solubility allows them to be easily integrated into biological systems and natural environments. The technology’s ability to produce high-quality nanomaterials in a continuous, controlled manner makes it a valuable asset for companies looking to lead in nanotechnology innovation.
Key Benefits for Medical, Environmental, and Tech Sectors
In the medical field, these water-soluble magnetic nanomaterials can enhance imaging techniques, such as MRI, and improve the precision of drug delivery systems, reducing side effects and improving patient outcomes. Environmental applications include using the nanomaterials for pollutant removal or water purification, offering an efficient way to address pressing environmental challenges. Additionally, companies in data storage and electronics can explore the use of magnetic nanomaterials to create smaller, more efficient devices. This technology’s flexibility and scalability make it a versatile tool for advancing multiple sectors.
Invest in the Future of Nanomaterials
Licensing this water-soluble magnetic nanomaterials growth technology positions your company at the forefront of nanotechnology innovation. By offering a scalable, continuous production process, your business can meet the growing demand for advanced materials across medical, environmental, and technological fields. This technology provides a strategic advantage for companies looking to improve product performance, enhance environmental sustainability, and drive breakthroughs in materials science.
- Abstract
- Claims
We claim:
Share
Title
Method for continuous growth of water-soluble magnetic nanomaterials
Inventor(s)
Yongfeng Zhao
Assignee(s)
Jackson State University
Patent #
20200262715
Patent Date
August 20, 2020
