Innovative Targeted Therapy for Immune Regulation
Introduction
This groundbreaking targeted degradation technology offers a precise approach to managing inflammatory diseases by degrading interleukin-1 receptor-associated kinase 4 (IRAK4) polypeptides, proteins linked to immune responses that can drive inflammation. Through targeted molecular action, this technology allows for precise modulation of immune signaling pathways, helping reduce inflammation with greater specificity and fewer side effects. Companies in the biotechnology, pharmaceutical, and immunology fields can leverage this technology to develop novel treatments for autoimmune diseases and inflammatory conditions, positioning themselves as leaders in advanced, targeted therapeutics.
The Challenge: Balancing Immune Response and Inflammation
Inflammatory diseases, including autoimmune disorders and certain cancers, are often driven by overactive immune responses that damage healthy tissues. Conventional therapies can suppress immune activity, but often lack the precision to target specific signaling molecules, leading to potential side effects and a weakened immune system. Providers and patients alike need solutions that can effectively target immune pathways, specifically molecules that contribute to inflammation, without compromising overall immune health.
Precision in Action: IRAK4 Targeted Degradation
This technology provides a novel approach by directly degrading IRAK4 polypeptides, selectively inhibiting a critical pathway in inflammation. By breaking down this specific protein, it reduces the overactive immune signaling that drives inflammatory diseases, offering a targeted solution that mitigates harmful immune activity while preserving broader immune function. The precise degradation mechanism offers significant advantages over traditional immune-modulating therapies, ensuring the immune system remains responsive to actual threats while reducing unnecessary inflammation. This approach allows healthcare providers to offer patients more effective treatments with a focus on safety and targeted action.
Key Benefits for Biotech and Pharma
For biotechnology and pharmaceutical companies, this targeted degradation technology represents a significant leap forward in immune regulation and inflammation control. It enables the development of specialized therapies for autoimmune diseases, oncology, and other conditions linked to inflammation, supporting the growing trend toward precision medicine. By integrating this approach into their pipeline, companies can enhance their portfolio with a unique, patient-focused technology that meets the needs of a diverse range of inflammatory diseases. The technology aligns well with market demand for safer, more precise immunotherapies, opening new opportunities in a highly competitive field.
Invest in the Future of Inflammation Therapy
Licensing this targeted immune regulation technology positions your company as a leader in innovative inflammation therapies. By providing a safe, precise solution for immune modulation, your business can support the development of therapies that are highly specific and patient-centered. This investment offers immense potential for companies committed to advancing patient care, reducing inflammation safely, and leading in the field of immunology and targeted treatment solutions.
- Abstract
- Claims
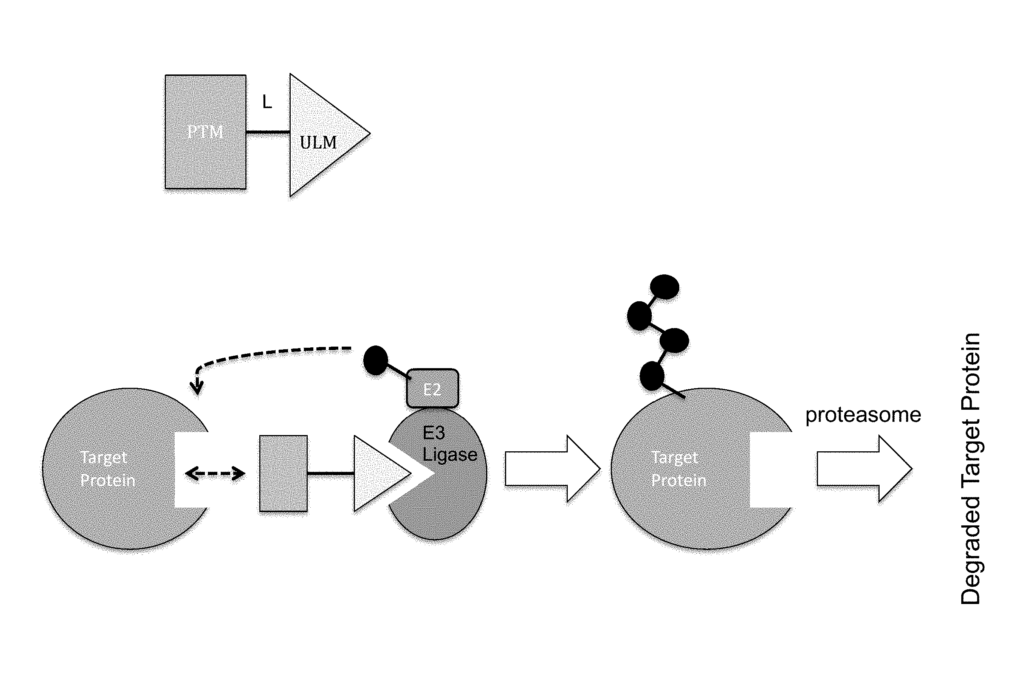
ULM-L-PTM,
-(AL)q-,
Share
Title
Compounds and methods for the targeted degradation of interleukin-1 receptor-associated kinase 4 polypeptides
Inventor(s)
Andrew P. Crew, Keith R. Hornberger, Kurt Zimmermann, Erika Araujo
Assignee(s)
Arvinas Inc, Arvinas Operations Inc
Patent #
20190151295
Patent Date
May 23, 2019





















































































































